Why The CDC Is Warning Americans To Stop Using EzriCare Artificial Tears
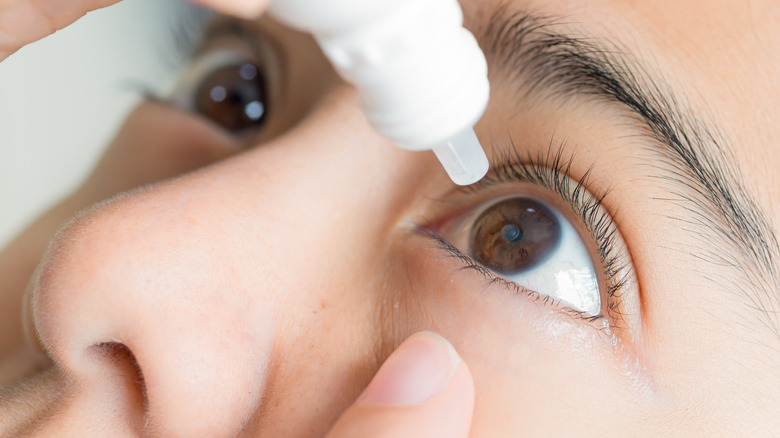
Individuals who experience dry eyes, whether from aging, medication side effects, allergies, a health condition, or more, may benefit from the use of artificial tears, reports the Mayo Clinic. Artificial tears are eye drops dispensed onto the outer eye that help hydrate our eyes. Additionally, some brands also support healing and prevent moisture evaporation.
Many kinds of artificial tear products contain preservatives, which protect against bacterial growth within the bottle (via Mayo Clinic). However, some brands do not contain preservatives, such as EzriCare Artificial Tears, according to a statement released by the American Academy of Ophthalmology (AAO). As outlined in the statement, a U.S. Centers for Disease Control and Prevention (CDC) investigation has commenced after about 50 cases of bacteria-related infections were detected across 11 states in connection with the product. Such states included California, Colorado, Connecticut, Florida, New York, Texas, and Washington, amongst others. Specimen samples were collected from patients over the course of seven months between May and December of 2022.
Bacteria detected in EzriCare artificial tears
Out of the roughly 50 identified cases, patient outcomes reported included permanent ocular infection-related vision loss, hospitalization, and one death due to bloodstream infection, as per the press release. Specifically, the CDC announced in the statement that antibiotic-resistant Pseudomonas bacteria were found within the EzriCare bottles (via CNN). Infection with the bacteria can develop into Pseudomonas aeruginosa infection which can make its way into the blood, stomach, lungs, tendons, and urinary tract, as well as external wounds or burns, reports WebMD. As a result, the CDC has advised both clinicians and patients to cease the use of the product for the duration of the investigation until the agency has finished laboratory testing.
EzriCare addressed the investigation in a statement via their website supporting the discontinuation of the product. They further explained that their artificial tear products are not designed, formulated, imported, or manufactured by the company itself. They also made clear that no formal recall has been issued at this time.