Abstract
Testing and treatment of tuberculosis infection (TBI) are recommended for people living with HIV (PLWH). We aimed to evaluate the care cascade of TBI treatment among PLWH in the era of antiretroviral therapy (ART) scale-up. This retrospective study included adult PLWH undergoing interferon-gamma release assay (IGRA)-based TBI screening during 2019–2021. PLWH testing IGRA-positive were advised to receive directly-observed therapy for TBI after active TB disease was excluded. The care cascade was evaluated to identify barriers to TBI management. Among 7951 PLWH with a median age of 38 years and CD4 count of 616 cells/mm3, 420 (5.3%) tested positive and 38 (0.5%) indeterminate for IGRA. The TBI treatment initiation rate was 73.6% (309/420) and the completion rate was 91.9% (284/309). More than 80% of PLWH concurrently received short-course rifapentine-based regimens and integrase strand transfer inhibitor (InSTI)-containing ART. The main barrier to treatment initiation was physicians’ concerns and patients’ refusal (85.6%). The factors associated with treatment non-completion were older age, female, anti-HCV positivity, and higher plasma HIV RNA. Our observation of a high TBI completion rate among PLWH is mainly related to the introduction of short-course rifapentine-based regimens in the InSTI era, which can be the strategy to improve TBI treatment uptake.
Similar content being viewed by others
Introduction
Tuberculosis (TB) is one of the most frequent infectious causes of mortality of people living with HIV (PLWH). Globally, there were an estimated 1.5 million TB deaths in 2020; of those, 14% were attributable to HIV/TB co-infection1. HIV infection increases both the risk of progression from primary infection with Mycobacterium tuberculosis to active TB disease and reactivation of latent TB2. The WHO guidelines therefore strongly recommend systematic testing and treatment of TB infection (TBI) for PLWH3,4. Although global progress in TB preventive treatment has been achieved among PLWH, the implementation of targeted testing and treatment of TBI remains suboptimal in Asia3. The barriers to optimizing management of TBI include unavailability of tests with better performance, limited access to TBI treatment, and low completion rates with 6 to 9 months of isoniazid regimens5.
Compared with 6- and 9-month daily isoniazid monotherapy (6H and 9H, respectively), short-course, rifamycin-based regimens have been shown to have a similar preventive effect with a higher completion rate and lower risk of adverse events6. Based on the growing evidence of benefits, the updated guidelines include 3 months of weekly rifapentine plus isoniazid (3HP) as preferred TBI treatment7. A clinical trial among PLWH further demonstrated that 1 month of daily rifapentine plus isoniazid (1HP) was also noninferior to the standard 9-month isoniazid for TBI treatment among PLWH who were receiving antiretroviral therapy (ART) consisting predominantly of non-nucleoside reverse-transcriptase inhibitor (NNRTI)-based regimens, but with a higher completion rate8.
Rifamycins are potent inducers of hepatic drug-metabolizing enzymes, leading to reduced plasma concentrations of co-administered antiretrovirals9. In pharmacokinetic studies, efavirenz or raltegravir can be used with weekly rifapentine without dose adjustment10,11; however, clearance of bictegravir (BIC), dolutegravir (DTG), elvitegravir (EVG) and cobicistat (COBI) will be significantly increased when co-administered with rifapentine12. Although clinical studies demonstrated that short-course rifapentine-based TBI treatment may not jeopardize the HIV control among PLWH taking BIC- or DTG-containing regimens13,14, clinicians may still be reluctant to prescribe rifapentine-based TBI treatment due to the concern of drug interactions5.
A previous systematic review of TBI care cascade among PLWH showed that the cumulative proportions of PLWH completing TBI testing and treatment were 83.6 and 41.9%, respectively15. Of the included 94,011 PWLH, only 43.6% received ART and 0.3% were treated with short-course rifamycin-based regimens. While ART scale-up has been implemented with the widespread use of integrase strand transfer inhibitor (InSTI) in recent years12,16, studies remain limited in PLWH who concurrently receive short-course rifamycin-based regimens and InSTI-containing ART. Taiwan has achieved the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 targets, with 90% of PLWH being aware of their HIV status, 94% of those diagnosed being on treatment, and 95% of those on treatment being virally suppressed in 2021. In this study, we aimed to evaluate the cascade of care and identify barriers to the treatment of TBI among PLWH in the era of InSTI-containing ART scale-up.
Methods
Study setting and population
Taiwan is a country of intermediate TB burden, with an incidence of 33.2 cases per 100,000 people in 202017. To achieve the WHO End TB Strategy targets, most of the populations at risk for TB identified according to the WHO TBI guidelines have been included for TBI testing and treatment in Taiwan. The national program for TBI has been expanded to include PLWH since 2019 by providing free-of-charge interferon-gamma release assay (IGRA) for PLWH seeking HIV care at the designated hospitals5,17. The participants testing positive for IGRA were advised to receive free-of-charge, directly-observed therapy (DOT) for TBI after active TB disease was excluded by review of clinical symptomatology and chest radiography7. The available TBI regimens included 1HP, 3HP, 3HR (three months of daily rifampicin plus isoniazid), 4R (four months of daily rifampicin), or 9H7. At the end of 2020, the program had been implemented in 33% (26/79) of designated hospitals for HIV care and enrolled 31% (10,563/33,699) of PLWH around Taiwan18.
This retrospective cohort study was conducted at 13 major designated hospitals for HIV care in Taiwan. During July 2019 to December 2021, PLWH aged 20 years or more who underwent IGRA-based TBI screening were included. PLWH were excluded from the study if they had had a previous history of TB or TBI treatment. For PLWH with IGRA positivity, eligibility for TBI treatment was evaluated by treating physicians. For those eligible for TBI treatment, TBI treatment regimens were chosen at the discretion of treating physicians. The main treatment considerations included treatment duration, adverse events, and drug interactions. Throughout the treatment course, in-person or smart-phone video-based DOT conducted by HIV case managers was used to monitor treatment adherence and report adverse events19. Clinical assessment and laboratory investigations (e.g. complete blood counts, aminotransferases, and bilirubin levels) were followed every 2–4 weeks in the first month and thereafter every 4 weeks until the end of treatment.
HIV medical care, including ART and monitoring of plasma HIV RNA load (PVL) and CD4 count, were provided to all PLWH according to the national HIV treatment guidelines20. Though both InSTI- and NNRTI-based regimens are the preferred first-line regimens in Taiwan, the proportion of PLWH initiating InSTI-based regimens increased sharply after the introduction of coformulated single-tablet InSTI-based regimens16.
TBI testing and treatment is a national public health policy and all PLWH gave written informed consent to participate in IGRA for TBI. This retrospective study was approved by the Research Ethics Committee or Institutional Review Boards of the participating hospitals (National Taiwan University Hospital [201003112R]; Far Eastern Memorial Hospital [105040-F]; Taoyuan General Hospital [TYGH103011]; Hsinchu Mackay Memorial Hospital [18MMHIS008e]; National Taiwan University Hospital Hsin-Chu Branch [105-017-F]; Chung Shan Medical University Hospital [CS14034]; Changhua Christian Hospital [160408]; National Cheng Kung University Hospital [B-BR-105-038]; Kaohsiung Medical University Hospital [KMUH-IRB20110040]) and informed consent to collection of clinical data for subsequent anonymized analysis was waived. The research was performed in accordance with relevant guidelines and the Declaration of Helsinki21.
TBI care cascade and barriers to TBI treatment
The TBI care cascade evaluated in this study included the following steps: (1) “Medical evaluation completed” as IGRA-positive (defined as interferon-gamma response to TB antigens being significantly above that to negative control) PLWH evaluated by review of clinical symptomatology and chest radiography; (2) “TBI diagnosed” defined as IGRA-positive PLWH without symptoms or image findings suggestive of active TB disease; (3) “Treatment initiated” as IGRA-positive PLWH having been prescribed any TBI treatment regimens; and (4) “Treatment completed” as IGRA-positive PLWH receiving at least 80% of doses within 120% of planned time; and reasons for treatment discontinuation or modification were recorded. The cumulative proportion retained in the care cascade was calculated by multiplying the proportion completing each step by the proportion completing the preceding step. The factors associated with treatment non-initiation and non-completion were analyzed.
Laboratory investigations
The QuantiFERON-TB Gold In-Tube (QFT-GIT) and QuantiFERON-TB Gold Plus (GFT-Plus) assays (Qiagen, Germantown, MD, USA) were performed by collecting whole blood into separate tubes, which included a Nil tube (negative control), a Mitogen tube (positive control), and one to two TB Antigen tubes22,23. IGRA was performed at each participating hospital using the same kit by following the instructions of manufacturer. While the test result was interpreted as positive when the TB Antigen minus Nil value was ≥ 0.35 IU/ml and ≥ 25% of the Nil value, the result was considered indeterminate if the Nil value was > 8.0 IU/mL (high Nil response) or the Mitogen minus Nil value was < 0.5 IU/mL (low Mitogen response). The sensitivity and specificity of QFT assay were 89.0 and 99.1%, respectively.
Statistical analyses
All statistical analyses were performed with the use of STATA software version 12.0 (Stata Corporation, College Station, TX). Categorical variables were analyzed using the chi-squared test or Fisher’s exact test and continuous variables were compared using the Wilcoxon-Mann–Whitney test. Logistic regression was used to clarify the factors associated with IGRA positivity. Variables with a P < 0.05 in univariate analyses were included in the maximum model for multivariate analyses, and a backward selection was used to determine the final model. All statistical tests were two-sided, and variables with P < 0.05 were considered significant.
Results
Characteristics of PLWH undergoing IGRA testing
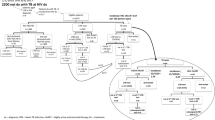
From July 2019 to December 2021, a total of 7951 adult PLWH without a previous history of TB or TBI treatment underwent IGRA testing (Fig. 1); the majority of them were male (96.0%) with a median age of 38 years (interquartile range [IQR], 31–45). The main route of HIV transmission was male-to-male sexual contact (79.5%), followed by illicit drug use (11.6%) and heterosexual sexual contact (6.9%). A high proportion (96.4%) of enrolled PLWH had achieved PVL < 200 copies/mm3 and the median CD4 count was 616 cells/mm3.
Overall, 420 (5.3%) tested positive for IGRA and 38 (0.5%) had indeterminate results. Compared with PLWH with IGRA negativity, those with IGRA positivity were older (median age, 43 vs. 38 years) and less likely to be male (92.4 vs. 96.2%). IGRA-positive PLWH were more likely to have acquired HIV through heterosexual contact (11.7 vs. 6.6%) and illicit drug use (25.0 vs. 10.8%), and to have had a history of incarceration (9.1 vs. 5.8%), anti-HCV positivity (28.3 vs. 16.2%), and higher baseline CD4 counts (median, 639 vs. 615 cells/mm3) (all P values < 0.05) (Table 1). In multivariable analysis, the independent factors associated with IGRA positivity were older age (per 1-year increase, adjusted odds ratio [AOR], 1.04; 95% confidence interval [CI], 1.02–1.05), illicit drug use (AOR, 2.11; 95% CI, 1.49–3.00), and higher baseline CD4 counts (per 10-cell/mm3 increase, AOR, 1.01; 95% CI, 1.00–1.01) (Table 2).
Of 38 PLWH with indeterminate IGRA results, 35 PLWH had low mitogen responses and 3 had high nil responses. PLWH with low mitogen responses had significantly lower baseline CD4 counts (median, 414 cells/mm3) compared to PLWH with positive and those with negative IGRA results (median, 639 and 615 cells/mm3, respectively) (Supplementary Table 1).
TBI treatment initiation
Overall, 73.6% (309/420) and 18.4% (7/38) of PLWH with positive IGRA and those with indeterminate IGRA results, respectively, initiated TBI treatments (Fig. 1). Of those IGRA-positive not receiving TBI treatment, the common reasons for not initiating TBI treatment were physicians’ concerns about adverse effects and drug-drug interactions and patients’ refusal (95/111, 85.6%) and loss to follow-up (8/111, 7.2%). However, there was no specific demographic variable independently predicting non-initiation (Supplementary Tables 3 and 4). Among 309 IGRA-positive PLWH initiating TBI treatment, the most common TBI regimens were 3HP (155/309, 50.2%), followed by 1HP (137/309, 44.3%) and 9H (17/309, 5.5%). The most frequent ART used was DTG-based regimens (166/309, 53.7%) and coformulated BIC, emtricitabine and tenofovir alafenamide (BIC/FTC/TAF) (105/309, 34.0%) (Supplementary Table 4).
TBI treatment completion
The overall completion rate was 91.9% (284/309): 93.4% in 137 PLWH receiving 1HP, 89.7% in 155 PLWH receiving 3HP, and 100% in 17 PLWH receiving 9H (Supplementary Table 4). Among 25 PLWH who failed to complete TBI treatment, 23 (92.0%) were due to adverse events and 2 (8.0%) due to drug interactions with methadone. The most common reasons of treatment non-completion included abnormalities in liver function tests (total bilirubin, 0.86–4.09 mg/dL; and alanine aminotransferase, 48–472 U/L) (6/29, 20.7%), urticaria (6/29, 20.7%), and nausea (6/29, 20.7%). The median time to discontinuation was 14 days (95% CI, 12–21 days). Compared with PLWH with treatment completion, PLWH with treatment non-completion were older (median age, 49 vs. 42 years), less likely to be male (76.0% vs. 94.4%), and more likely to have acquired HIV through illicit drug use (36.0 vs. 19.7%), have anti-HCV positivity (40.0 vs. 11.6%), and receive DTG-containing regimens (80.0 vs. 51.4%) (Supplementary Table 2). In multivariable analysis, the predictors of treatment non-completion were older age (per 1-year increase, AOR, 1.07; 95% CI, 1.02–1.11), not male sex (AOR for male, 0.27; 95% CI, 0.09–0.85), anti-HCV positivity (AOR, 4.45; 95% CI, 1.73–11.47), and PVL before TBI treatment (per 1-log10 copies/mL increase, AOR, 1.78; 95% CI, 1.07–2.95) (Table 3).
TBI care cascade
Among IGRA-positive PLWH, the cumulative proportions of medical evaluation completed, TBI diagnosed, treatment initiated, and treatment completed were subsequently 100% (420/420), 99.0% (416/420), 73.6% (99.0% × [309/416]) and 67.6% (73.6% × [284/309]), respectively (Fig. 2). The main gap of care cascade occurred at the step of treatment initiation (25.7%, 107/416).
Discussion
In this study conducted during the roll-out of TBI screening and treatment in Taiwan, we found the overall prevalence of TBI was 5.3% among PLWH in the modern era of ART and declining TB incidence. The TBI treatment uptake and completion rates in this cohort were 73.6 and 91.9%, respectively, when short-course rifamycin-based regimens available. More than 80% of the included PLWH were concurrently receiving short-course rifapentine-based regimens and InSTI-containing ART and the major gap of engagement in care was related to TBI treatment initiation.
Targeted testing and treatment for TBI have been prioritized for PLWH and showed cost-effectiveness in countries with different TB endemicities24,25. The United States reported the lowest TB incidence rate in the world (2.7 per 100,000 population in 2019) and estimated an TBI prevalence rate to be 4.7% among 1510 PLWH26. Hong Kong, as a city with intermediate TB burden, had an TBI prevalence of 26.2% among 2412 PLWH27. In Taiwan, a previous study including 909 PLWH during 2008–2010 demonstrated that the TBI prevalence was 36.6% in men who have sex with men (MSM), 12.7% in heterosexuals, and 50.7% in injecting drug users28. Another study of 608 PLWH who were mainly MSM during 2011–2013 found a lower TBI prevalence (10.5%)29. Compared with the PLWH included in the earlier study (median CD4, 441–578 cells/mm3; proportion of PVL < 50 copies/mm3, 50.0%), our study cohort in the era of ART scale-up had improved immunologic and virologic characteristics (median CD4, 616 cells/mm3; proportion of PVL < 50 copies/mm3, 92.2%)29, which could reduce the probability of both false-negative and indeterminate IGRA results30. Therefore, the even lower TBI prevalence shown in our study might reflect the steadily declining TB incidence in Taiwan17. The findings of association of older age, illicit drug use, and increased CD4 counts with positive TBI test results in this study are in line with those in other studies3,29,31.
In a recent meta-analysis, the estimated rates of TBI treatment initiation and completion among PLWH with positive TBI tests were 86.3 and 69.4%, respectively. The main losses of the care cascade were in the provider recommendation and TBI treatment completion. The reported barriers included adverse events, pill burden, drug interactions, and lack of knowledge among healthcare workers and patients. In the 70 included cohorts, only one primarily used rifamycin-based short-course regimens (3–4 months of rifampicin plus isoniazid)15. In general population, short-course preventive therapy regimens facilitated treatment uptake and enhance treatment completion for individuals with TBI6. The extension of PREVENT TB trial enrolled 399 PLWH and showed a higher completion rate of 3HP when compared with 9H (89 vs. 64%)32. The BRIEF TB trial that enrolled 3000 PLWH mainly receiving NNRTI-based antiretroviral therapy also demonstrated a higher completion rate of 1HP compared with 9H (97 vs. 90%)8. While the treatment completion rate in our cohort study was consistent with those observed in the clinical trial settings8,32, the findings of factors identified to be associated with treatment non-completion imply the need for carefully monitoring adverse events in older PLWH, and those with viral hepatitis and virological non-suppression. Individuals with older age and viral hepatitis are more likely to develop adverse drug reactions during TBI treatment, particularly hepatotoxicity. Virological non-suppression could be related to poor linkage to care and adherence to medicine, resulting in treatment non-completion. Therefore, treatment completion may be enhanced by counseling, support, and comprehensive follow-up.
Despite the adoption of short-course rifapentine-based regimens during the launch of TBI testing and treatment program, the treatment initiation rate in our study (73.6%) was still lower than that in previous studies15. While co-formulated single-tablet InSTI-containing regimens have become the recommended first-line HIV treatment regimens12, concerns about adverse effects and drug-drug interactions cause the hesitancy to initiate TBI treatment in this cohort. Indeed, co-administration of rifapentine significantly decreases the plasma concentrations of InSTIs due to rifapentine-mediated CYP3A and UGT1A1 induction33. In a phase 1/2 trial conducted among 61 PLWH concurrently receiving DTG-containing regimens and 3HP, a 36% increase in DTG clearance was observed; however, only one had trough concentration below the 90% maximal inhibitory concentration for DTG and all participants were able to maintain HIV viral suppression during 3HP treatment13. In another study of 48 PLWH concurrently receiving BIC/FTC/TAF and 1HP, the proportion of BIC trough concentrations above the 95% effective concentration dropped to 37%; however, more than 90% of participants were able to maintain HIV viral suppression during and after 1HP treatment14. Although the findings suggest that viral suppression still could be maintained and no cases of virologic failure occurred after TBI treatment among PLWH receiving InSTI-containing regimens, transient HIV viremia was noted during TBI treatment due to a significant decrease in BIC trough concentration. Considering that viral blips are not predictive of virologic failure12,34, both BIC- and DTG-containing regimens could be considered to be co-administered with 1HP or 3HP under virologic monitoring, though more clinical and pharmacokinetic studies are warranted to confirm the findings.
Our study has several limitations. First, the study was conducted among 23.6% (7,951/33,699) of PLWH around Taiwan; therefore, the prevalence and associated factors of TBI reported in this study may not be generalized to a larger population. Second, the absence of clinical symptoms or radiographic abnormalities may not exclude incipient and subclinical TB. Furthermore, the indeterminate IGRA results cannot provide useful information about the likelihood of M. tuberculosis infection, but only a few PLWH with indeterminate results underwent repeat IGRA tests till the end of the study. Lastly, the detailed reasons for not considering to initiate TBI treatment by both IGRA-positive PLWH or care providers could not be clearly defined in this retrospective cohort study and concerns might differ across physicians. More qualitative studies are needed to better understand these concerns and more investigations on the safety profile and maintenance of viral suppression among PLWH receiving TBI treatment are needed to alleviate the concerns.
Conclusions
The high completion rate among those mainly receiving short-course rifapentine-based regimens in the InSTI era indicated the current strategy to be effective in improving the TBI treatment for PLWH.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available, since the participants did not consent to the sharing of data with third parties, but are available from the corresponding author on reasonable request.
References
World Health Organization. Global tuberculosis report 2021. https://www.who.int/publications/i/item/9789240037021 (2021).
Kwan, C. K. & Ernst, J. D. HIV and tuberculosis: A deadly human syndemic. Clin. Microbiol. Rev. 24, 351–376. https://doi.org/10.1128/CMR.00042-10 (2011).
World Health Organization. Latent tuberculosis infection: Updated and consolidated guidelines for programmatic management. http://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/ (2018).
Goletti, D., Delogu, G., Matteelli, A. & Migliori, G. B. The role of IGRA in the diagnosis of tuberculosis infection, differentiating from active tuberculosis, and decision making for initiating treatment or preventive therapy of tuberculosis infection. Int. J. Infect. Dis. https://doi.org/10.1016/j.ijid.2022.02.047 (2022).
Paton, N. I. et al. Diagnosis and management of latent tuberculosis infection in Asia: Review of current status and challenges. Int. J. Infect. Dis. 87, 21–29. https://doi.org/10.1016/j.ijid.2019.07.004 (2019).
Hamada, Y., Ford, N., Schenkel, K. & Getahun, H. Three-month weekly rifapentine plus isoniazid for tuberculosis preventive treatment: A systematic review. Int. J. Tuberc. Lung Dis. 22, 1422–1428. https://doi.org/10.5588/ijtld.18.0168 (2018).
World Health Organization. WHO consolidated guidelines on tuberculosis. https://www.who.int/publications/i/item/9789240001503 (2020).
Swindells, S. et al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N. Engl. J. Med. 380, 1001–1011. https://doi.org/10.1056/NEJMoa1806808 (2019).
Regazzi, M., Carvalho, A. C., Villani, P. & Matteelli, A. Treatment optimization in patients co-infected with HIV and Mycobacterium tuberculosis infections: Focus on drug-drug interactions with rifamycins. Clin. Pharmacokinet. 53, 489–507. https://doi.org/10.1007/s40262-014-0144-3 (2014).
Podany, A. T. et al. Efavirenz pharmacokinetics and pharmacodynamics in HIV-infected persons receiving rifapentine and isoniazid for tuberculosis prevention. Clin. Infect. Dis. 61, 1322–1327. https://doi.org/10.1093/cid/civ464 (2015).
Weiner, M. et al. Pharmacokinetic interaction of rifapentine and raltegravir in healthy volunteers. J. Antimicrob. Chemother. 69, 1079–1085. https://doi.org/10.1093/jac/dkt483 (2014).
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/whats-new-guidelines (2019).
Dooley, K. E. et al. Once-weekly rifapentine and isoniazid for tuberculosis prevention in patients with HIV taking dolutegravir-based antiretroviral therapy: A phase 1/2 trial. Lancet HIV 7, e401–e409. https://doi.org/10.1016/S2352-3018(20)30032-1 (2020).
Liou, B. H. et al. Short-course daily isoniazid and rifapentine for latent tuberculosis infection in people living with HIV who received coformulated bictegravir/emtricitabine/tenofovir alafenamide. J. Int. AIDS Soc. 24, e25844. https://doi.org/10.1002/jia2.25844 (2021).
Bastos, M. L., Melnychuk, L., Campbell, J. R., Oxlade, O. & Menzies, D. The latent tuberculosis cascade-of-care among people living with HIV: A systematic review and meta-analysis. PLoS Med. 18, e1003703. https://doi.org/10.1371/journal.pmed.1003703 (2021).
Huang, Y. C. et al. Short-term outcomes of rapid initiation of antiretroviral therapy among HIV-positive patients: Real-world experience from a single-centre retrospective cohort in Taiwan. BMJ Open 9, e033246. https://doi.org/10.1136/bmjopen-2019-033246 (2019).
Taiwan Centers for Disease Control. Taiwan tuberculosis control report 2020. https://www.cdc.gov.tw/InfectionReport/Info/uKmf00HvSmkNaX9lNY-raQ?infoId=QE5nPSOHtxLFdGMvUh_LPw (2022).
Liao, S. C., Chan, P. C., Feng, C. F., Lee, C. C. & Liao, S. C. Scaling-up of latent TB infection diagnosis and treatment among PLHIV in Taiwan from zero. In The 52th World Conference on Lung Health. E-poster no. EP-10-189 (2021).
Story, A. et al. Smartphone-enabled video-observed versus directly observed treatment for tuberculosis: a multicentre, analyst-blinded, randomised, controlled superiority trial. Lancet 393, 1216–1224. https://doi.org/10.1016/S0140-6736(18)32993-3 (2019).
Taiwan AIDS Society. Guidelines for diagnosis and treatment of HIV/AIDS 6th edn. http://www.aids-care.org.tw/journal/treatment.php (2020).
World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310, 2191–2194. https://doi.org/10.1001/jama.2013.281053 (2013).
QuantiFERON®-TB Gold (QFT®) ELISA (Package Insert) Qiagen (2017).
QuantiFERON®-TB Gold Plus (QFT®-Plus) (Package Insert) Qiagen (2021).
Jo, Y. et al. Model-based cost-effectiveness of state-level latent tuberculosis interventions in California, Florida, New York and Texas. Clin. Infect. Dis. 73, e3476–e3482. https://doi.org/10.1093/cid/ciaa857 (2021).
Capocci, S. et al. Decreasing cost effectiveness of testing for latent TB in HIV in a low TB incidence area. Eur. Respir. J. 46, 165–174. https://doi.org/10.1183/09031936.00067114 (2015).
Pettit, A. C. et al. Optimal testing choice and diagnostic strategies for latent tuberculosis infection among U.S.-born people living with HIV. Cli Infect. Dis. 73, e2278–e2284. https://doi.org/10.1093/cid/ciaa1135 (2020).
Wong, N. S. et al. Latent tuberculosis infection testing strategies for HIV-positive individuals in Hong Kong. JAMA Netw. Open 2, e1910960. https://doi.org/10.1001/jamanetworkopen.2019.10960 (2019).
Yang, C. H. et al. Strategy to better select HIV-infected individuals for latent TB treatment in BCG-vaccinated population. PLoS ONE 8, e73069. https://doi.org/10.1371/journal.pone.0073069 (2013).
Sun, H. Y. et al. Risk of active tuberculosis in HIV-infected patients in Taiwan with free access to HIV care and a positive T-Spot. TB test. PLoS ONE 10, e0125260. https://doi.org/10.1371/journal.pone.0125260 (2015).
Raby, E. et al. The effects of HIV on the sensitivity of a whole blood IFN-gamma release assay in Zambian adults with active tuberculosis. PLoS ONE 3, e2489. https://doi.org/10.1371/journal.pone.0002489 (2008).
Wong, N. S. et al. A longitudinal study on latent TB infection screening and its association with TB incidence in HIV patients. Sci. Rep. 9, 10093. https://doi.org/10.1038/s41598-019-46570-5 (2019).
Sterling, T. R. et al. Three months of weekly rifapentine and isoniazid for treatment of Mycobacterium tuberculosis infection in HIV-coinfected persons. AIDS 30, 1607–1615. https://doi.org/10.1097/QAD.0000000000001098 (2016).
Lu, C. H. et al. Pharmacokinetic drug interactions of integrase strand transfer inhibitors. Curr. Res. Pharmacol. Drug Discov. 2, 100044. https://doi.org/10.1016/j.crphar.2021.100044 (2021).
Chen, G. J. et al. Low-level viremia and virologic failure among people living with HIV who received maintenance therapy with co-formulated bictegravir, emtricitabine and tenofovir alafenamide versus dolutegravir-based regimens. Int. J. Antimicrob. Agents https://doi.org/10.1016/j.ijantimicag.2022.106631 (2022).
Acknowledgements
The authors thank all study members participating in the Taiwan HIV Study Group.
Author information
Authors and Affiliations
Consortia
Contributions
C.Y.C., P.L.L., and C.C.H. managed and supervised the study. K.Y.L., C.Y.C., P.L.L., and C.C.H. designed the study. K.Y.L., C.J.Y., H.Y.S., Y.T.L., B.H.L., I.M.H., T.C.C., S.H.H., C.Y.L., C.S.T., C.Y.L., H.Y.C., C.Y.C., and P.L.L. were involved in collection and assembly of clinical data. K.Y.L., C.Y.C., P.L.L., and C.C.H. analyzed data and interpreted results. The first draft was written by K.Y.L., C.Y.C., P.L.L., and C.C.H. with substantial revisions and input from all authors. All authors reviewed and approved the final version of this report.
Corresponding author
Ethics declarations
Competing interests
C.C.H. has received research support from Merck, Gilead Sciences, and ViiV and speaker honoraria from Gilead Sciences and ViiV, and served on advisory boards for Gilead Sciences and ViiV. H.Y.S. has received research support from Gilead Sciences. Other authors have no competing interest to disclose.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, KY., Yang, CJ., Sun, HY. et al. Care cascade of tuberculosis infection treatment for people living with HIV in the era of antiretroviral therapy scale-up. Sci Rep 12, 16136 (2022). https://doi.org/10.1038/s41598-022-20394-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-20394-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.