Abstract
Background/Objectives
Despite a significant disease burden and potential to cause blindness, primary angle closure disease (PACD) does not have a population-based screening programme. Biometric indices using ultrasound A-scan is a potential tool for glaucoma case-detection. Given that genetic and environmental factors influence these parameters and paucity of data on their discrimination thresholds in Indian populace, we conducted a matched case-control study to determine the biometric indices and their discrimination thresholds associated with PACD.
Methods
We studied 172 eyes of 86 participants (43 cases; 43 controls). We compared the following biometric parameters of cases (PACD, occludable angle ≥180° ± raised intraocular pressure) with age and gender-matched controls (1:1): Anterior chamber depth (ACD), lens thickness (LT), axial length (AXL), lens position (LP), relative lens position (RLP), lens axial factor (LAF), simple crowding value (Cs), ACD/AXL). We performed conditional logistic regression (to identify factors associated with PACD) and Receiver operating characteristic (ROC) analysis (to determine discrimination thresholds).
Results
Reduced ACD (Adj OR 0.01; 95% CI: 0.0003–0.15, p < 0.001) and increased LT (Adj OR 10.3; 95% CI:2.42–43.93, p < 0.001) were associated with PACD. On ROC analysis, ACD, Cs, and ACD/AXL had optimum sensitivity/specificity at ≤3.015, ≥0.056 and ≤0.1303, respectively. ACD (88.4%) and Cs (94.2%) had highest sensitivity and specificity, respectively.
Conclusion
Ultrasonic biometric parameters differed significantly between PACD and controls. ACD and Cs (at discrimination thresholds of ≤3.015 mm and ≥ 0.056, respectively) using ultrasound A-scan could be a potential tool for PACD case-detection that requires evaluation of its diagnostic yield and cost-effectiveness.
Similar content being viewed by others
Introduction
Glaucoma is the second leading cause of blindness worldwide and an estimated 12 million people are blind due to the disease [1]. Globally, by 2040, the number of people affected by glaucoma is projected to increase to about 112 million, and South Central Asia is projected to record the steepest increase compared to other Asian sub-regions [2, 3]. In India, one in every eight persons aged ≥40 years has or is at risk of glaucoma [4]. Primary angle closure disease (PACD) is estimated to affect 27.6 million persons in some form or the other [4]. A surge in glaucoma cases is expected in the Indian subcontinent owing to the accelerated growth of population over 40 years of age, overburdening the scarce health resources [5]. Primary angle closure glaucoma (PACG) is more blinding than primary open-angle glaucoma, especially in the Indian and Chinese populations [4]. The disease is largely asymptomatic and chronic in India [6].
Blindness from primary angle closure glaucoma can be prevented by established treatments such as laser iridotomy and removal of the crystalline lens [7, 8].
Despite the high disease burden and availability of amenable treatment options, glaucoma was not included in the initial 5-year priority list of vision 2020 mainly due to a lack of practical and cost-effective population-based strategies, to prevent glaucoma-blindness [9]. Currently it is diagnosed by opportunistic screening [10]. A better understanding of PACD characteristics and its epidemiology, especially in Asia, has offered the potential for screening of risk factors so that timely prophylaxis can be implemented to prevent blindness [9, 11].
Although gonioscopy remains the gold standard for diagnosing angle closure, it is subjective and moderately reproducible, thus unsuitable for mass screening [6, 12]. Furthermore, routine ophthalmic examination in India, seldom involves gonioscopy, resulting in a low PACD detection rate [6, 10]. The flashlight test, a commonly used screening tool in the field, has a low positive predictive value (43.5–45%) [13]. Van Herick’s test is known to miss a significant number of angle closures and incorrectly identify around 1 in 8 open-angle eyes as closed, even in experienced hands [14]. The newer and expensive non-contact techniques such as the IOL Master, scanning peripheral anterior chamber depth (ACD) analyser and anterior segment optical coherence tomography have poor to moderate specificity (55.4–84%), and are not suitable for mass screening [12, 15]. Over diagnosing PACD (high false positives) will result in excessive referrals and overtreatment of the condition. Ultrasound biomicroscopy permits a detailed evaluation of the angle, but the need for a water bath, supine position, and greater skill of the examiner, makes it an inconvenient screening tool [16]. Evidence suggests that integration of genetic screening is not advantageous in identifying PACD beyond what is achieved with anatomical ocular parameters [17]. Thus, mass screening for PACD remains challenging due to technical difficulties, cost and scalability.
In contrast to various screening methods described above, the A-scan ultrasound machine is relatively inexpensive, portable equipment and an integral part of any cataract treating facility. A technician can be trained with relative ease to obtain accurate scans [18]. Previous studies have explored the association of the following biometric indices with a spectrum of PACD: ACD, axial length (AXL), ACD/AXL, lens thickness (LT), lens axial factor (LAF), relative lens position (RLP) and simple crowding value (Cs) [16, 19, 20]. With an appropriate cut-off point having optimal sensitivity and specificity, these indices can be used as potential surrogates to detect PACD. A few studies have determined these cut-off values among East Asian and Iranian populations [20,21,22,23]. Although certain studies from India have assessed few biometric indices, there is a lack of data on optimal cut-offs (discrimination thresholds) to differentiate individuals with and without PACD [24, 25].
Given that genetic and environmental factors influence the ocular biometric parameters [26, 27] and paucity of data in the Indian populace, we conducted a hospital-based case-control study in a coastal town of South India, with the following objectives
-
1.
To determine the ultrasonic biometric indices associated with PACD and
-
2.
To determine the optimal discrimination thresholds of ultrasonic biometric indices to detect PACD.
Methodology
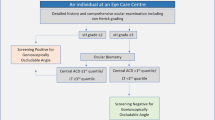
After obtaining approval from Institutional Ethics Committee (Reference number YEC2/258), we conducted this case-control study in the department of ophthalmology of a tertiary care hospital from February to March 2020. The study adhered to the tenets of the Declaration of Helsinki. We obtained written informed consent from the study participants.
Inclusion criteria
All consecutive patients ≥18 years who consented to take part in the study and who fulfilled the criteria for cases and controls were enrolled.
Cases
PACD was defined as those with occludable angle (non-visualisation of posterior trabecular meshwork for ≥180°), on gonioscopy without indentation or manipulation, with or without evidence of raised intraocular pressure (IOP). Undilated fundus examination was performed using indirect ophthalmoscopy with 78D lens wherever possible. Cases who had undergone laser peripheral iridotomy (LPI) were also included as existing evidence suggests that LPI does not affect the biometric variables of the eye including central ACD, LT, AXL [7].
Controls
Subjects who had come for a routine eye examination, correction of refractive errors, lid or ocular surface disorders, or any other issue but with otherwise healthy eyes were considered as controls after matching for age (same calendar year of birth) and gender. One control was selected for each case.
Exclusion criteria
All cases of secondary angle closure glaucomas, advanced cataracts (≥grade III cataracts), previous ocular trauma, intraocular surgeries (other than LPI) or any other condition that prevented gonioscopic examination were excluded.
Sample size
Based on reported mean LT in cases (4.52 ± 0.515) and controls (4.235 ± 0.44) [24, 28,29,30], a sample of 43 participants for each group was required, for detecting a true mean difference of 0.285 (i.e. 4.52–4.235) with 80% power and 5% (two sided) level of significance.
Data collection
Demographic data included age and gender. All patients underwent a thorough ophthalmic examination including best corrected visual acuity, slit lamp bio-microscopy, Goldmann applanation tonometry. Wherever possible, we performed an undilated indirect fundus examination using a 78D lens. One of the authors (AD) measured the ultrasonic biometric variables using A-Scan ultrasonography (Echorule Pro, Biomedix Optotechnik & Devices, Bengaluru, India). After anaesthetising the cornea with 0.5% Proparacaine (0.5% Paracaine, Sunways India Pvt Ltd, Ahmedabad, India), A-scan was performed without applying any pressure on the cornea with the subject’s gaze fixed on a distant target. We took three successive readings until the standard deviation of AXL and ACD were within 0.3 mm and 0.1 mm, respectively. The different ultrasonic biometric variables included LT, ACD, and AXL.
We calculated the following composite indices: Lens position (LP) = ACD + 0.5 LT; Relative lens position (RLP) = (LP/AXL); Lens axial factor (LAF) = (LT/AXL) × 10; Simple crowding value (Cs) = (LT − ACD)/AXL [19, 20, 31]. A senior ophthalmologist (SSav) performed the gonioscopy. We used Goldmann’s 3-mirror gonio-lens (Volk optics, Ohio, USA) under standardised conditions namely dim illumination, narrow slit beam with the patient’s gaze in primary position.
Statistical analysis
We calculated mean value ± standard deviation for all continuous data. An Independent two-sample t-test was used to compare continuous data between both eyes and also between cases and controls. A p value < 0.05 (two-tailed) was considered statistically significant. Biometric parameters with statistically significant differences between cases and controls were used to build a conditional logistic regression analysis for matched case control study. We plotted receiver operating characteristic (ROC) curves for the independent and composite factors to assess PACD. The area under the ROC curve (AUROC), sensitivity, specificity, and discrimination thresholds were calculated. The most optimal sensitivity/specificity relationship (discrimination thresholds) was determined using Youden’s index [(Sensitivity + specificity)−1] [32]. We used Stata 15 software (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC.) for analysis.
Results
A total of 62 patients were screened for eligibility and 43 cases (86 eyes) were included (five no consent; ten not eligible and four non-cooperative for Gonioscopy/A-scan). A total of 51 control were approached and 43 (86 eyes) were included (four no consent; four non-cooperative for gonioscopy/A-scan). The mean age of the participants was 53.47 ± 9.1 years. Most of the participants (72, 83.72%) were females. Observed differences in mean ACD, AXL, and LT among right and left eyes in cases and controls were not statistically significant.
On independent sample t-test, the following factors were significantly different among cases and controls: ACD, LT, AXL, LP, RLP, LAF, Cs, ACD/AXL (Table 1). The mean IOP among cases was significantly higher (20.26 mmHg ± 5.04) than controls (11.95 mmHg ± 1.27) with p < 0.001. Twenty five cases had IOP > 21 mmHg (range: 22–32 mmHg).
On conditional logistic regression, shorter ACD and increased LT were significantly associated with PACD (Table 2). Every millimetre increase in ACD was associated with 0.01 times lower odds (95% CI: 0.0003–0.15; p < 0.001) of PACD. Similarly, every millimetre increase in LT was associated with 10.3 times higher odds of PACD (95% CI: 2.42–43.93; p < 0.001).
On ROC curves, ACD, simple crowding value (Cs), and ACD/AXL had optimum sensitivity and specificity with discrimination thresholds of ≤3.015, ≥0.056, and ≤0.1303, respectively (Table 3 and Fig. 1).
Discussion
We found that cases of PACD had significantly shallower anterior chamber and thicker lens (LT) compared to age and gender-matched controls. Eyes with PACD have a disproportionately larger lens compared to their AXL. This is represented by a higher LAF value which was reflected in our study (LAF of cases 1.95, controls 1.7) [19]. Eyes with PACD also had more anteriorly situated lenses suggested by the smaller LP and RLP values in the PACD group (LP 4.93 ± 0.41 vs 5.22 ± 0.37; RLP 0.218 ± 0.016 vs 0.226 ± 0.016) as compared to the controls. The number of lens fibres in the crystalline lens increases as we age and results in increase in LT. In this study we have tried to negate the effect of age and cataract status on the LT by age-matching and by excluding participants with ≥grade III cataracts. Niu et al described simple crowding value (Cs) as a composite factor of LT, ACD, and AXL associated with angle closure [20]. A larger Cs value indicates a more crowded angle. We found a significantly larger Cs value in the PACD group compared to normal (0.08 ± 0.03 vs 0.03 ± 0.02).
On conditional logistic regression, we found that the adjusted odds of PACD were highest for shallower ACD (after adjusting for LT and AXL). ACD is the single most important factor which differentiates PACD from normal eyes [20]. The diagnostic value of ACD for identifying the risk of angle closure has been studied previously [22, 23, 33]. However, the cut-off values of the ocular biometric parameters differ significantly among different ethnicities as well as different regions. Genetic and environmental factors are known to influence the ocular biometric parameters [26, 27]. It is therefore pertinent to determine the region and population-specific optimal discrimination thresholds for the biometric indices.
On ROC analysis, ACD had the highest sensitivity (88.4%) at an optimal cut-off value of ≤3.015 mm. We considered the distance from the anterior corneal epithelium to the anterior lens surface as the ACD measurement. ACD, therefore, included the central corneal thickness (CCT). The “true” ACD however is the axial distance from the corneal endothelium to the anterior lens surface and does not include CCT (“true” ACD = ACD-CCT) [34]. We did not measure the CCT in our study. The average CCT in our population is about 0.536 mm [35]. Hence, if we assume a CCT of 0.536 mm, the “true” ACD cut-off values would be ≤2.479 mm. Many studies do not specify if the ACD was measured from the corneal epithelium or endothelium. The ACD values reported range from 1.53 to 3 mm [24, 25, 36]. The definition of cases may be variable in different studies (non-visualisation of posterior trabecular meshwork ≥180 degrees vs 270 degrees), contributing to differences in cut-off values [22, 23]. We did not perform indentation Gonioscopy to rule out synaechial angle closure nor did we attempt to categorise our cases into Primary angle closure suspect (PACS), Primary angle closure (PAC) and PACG. It is known that there is a linear trend towards more shallow ACD in cases with PACG vs those with PAC vs PACS [37]. The varying accuracies of different measurement techniques (handheld/immersion ultrasound A-scan/optical pachymeter) could also contribute to ACD variations [23].
We found that simple crowding value (Cs) had the highest specificity (94.2%) at an optimal cut-off of ≥0.056. Nui et al reported the Cs cut-off value as ≥0.11 in a study performed using an optical biometer, on Han Chinese patients with acute angle closure glaucoma and not on PACD cases. This could explain the variation in values. ACD/AXL had moderate sensitivity (81.4%) and specificity (86%).
Evidence suggests that ocular biometric parameters can be used to predict the risk of PACD [27]. We found ACD and Cs as potential predictors which can be used for mass screening of our population. Currently, in a developing country like India, opportunistic screening when the patient presents to an eye clinic, is the best approach for glaucoma disease detection [10]. The opportunity is however underutilized due to the time-consuming and skilled nature of Gonioscopic examination. Also, the utilisation of gonioscopy as a mass screening tool appears unrealistic to a large extent. These hurdles in PACD screening can be overcome by the utilisation of A-scan.
Do we need to screen and treat PACS?
Two large clinical trials, the Zhongshan Angle Closure Prevention (ZAP) Trial and the Singapore Asymptomatic Narrow Angles Laser Iridotomy Study have attempted to answer this important question [38, 39]. They concluded that although the trials showed that LPI almost halved the risk of progression of PACS (to PAC/PACG/Acute angle closure), interventions for community-level active case detection of PACS and LPI may not be recommended at a programmatic level in view of lower rates of progression in their trial cohorts. The results of this trial need to be re-appraised in the Indian context. In the Indian population, PACS has been shown to progress to PAC among 22% cases over a span of 5 years [40] as compared to 4.05% over 6 years in the ZAP trial control arm (7.97 per 1000 eye-years) and 9.4% (21.84 per 1000 eye-years) in the Singapore study control arms. Also, Indian eyes are more prone to progression to PACG from PAC (28.5% in 5 years) [41] as compared to Chinese (4.1% in 6 years) [42]. Hence, in view of the rapid progression of the disease in Indian eyes, the cost-effectiveness of PACS screening and LPI need to be re-assessed in the Indian scenario.
Also, one in every twelve adults (more than 74 million) in India have diabetes [43] and need repeated dilated fundus examination for diabetic retinopathy screening. Dilatation again can precipitate an attack of angle closure glaucoma in PACS [38]. This again illustrates the point that screening for PACS and LPI might still have a role to play in Indian scenario.
India has a robust cataract surgical programme [11]. India is one of the well-performing countries with respect to achieving the target cataract surgery rate (CSR) (i.e. number of cataract surgeries performed per million population). In the year 2018–19, around 6.6 million cataract surgeries were performed, achieving the target CSR [44]. In 2019–20, 18,306 eye screening camps were conducted across India [44]. In a resource-limited country like India, utilising equipment that is available and widely used for cataract surgery, for screening PACD, would be a good option. There is also evidence suggesting that clear lens extraction is a cost-effective treatment of PAC and PACG [8]. Hence, these subgroups of PACD have emerged as newer indications for cataract surgery. With a common treatment protocol for both the diseases (cataract and PAC/PACG), it is logical to integrate PACD screening using ultrasound A-scan into the existing cataract screening programme.
Strengths
Although biometric parameters have been studied in the context of PACD, there is a dearth of evidence for population-specific optimal discrimination threshold values in our population. This study attempts to fill in this gap in knowledge.
Limitations
Study findings apply to our population or population with similar racial, ethnic and environmental factors. As we did not perform indentation gonioscopy and visual fields, we did not classify PACD as PACS, PAC and PACG. We did not measure the “true” ACD. The outcome assessor measuring the ultrasonic biometric parameters was not masked to the gonioscopic findings. However, measurement bias was reduced by repeated measurements by a single investigator to obtain values that were within a known acceptable limit of standard deviation. The positive predictive value (the proportion of individuals with a positive result who actually have the disease) is dependent on the prevalence of the condition being tested. Thus, the true utility of this tool in community-level screening needs to be assessed by a large field-based diagnostic accuracy study. Such a study will also be able to address the concerns associated with the sample size and hospital-based nature of this study.
Conclusion
The ultrasonic biometric parameters differed significantly between PACD and normal eyes. ACD and Cs, at discrimination thresholds of ≤3.015 mm and ≥0.056, respectively, using hand-held ultrasound A-scan are potential tool for PACD case-detection in our population. The diagnostic yield and cost-effectiveness of incorporating A-scan into ongoing cataract screening programmes need further evaluation.
Summary
What was known before
-
Ocular biometric parameters differ significantly among PACD and normal eyes. Existing screening tools for PACD are not suitable due to various limitations.
What this study adds
-
Discrimination thresholds (cut-off values) of various ocular biometric parameters to differentiate PACD and normal eyes. ACD and Cs with discrimination thresholds could be a potential screening tool for PACD.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51.
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology. 2020;121:2081–90. https://pubmed.ncbi.nlm.nih.gov/24974815/
Chan EWE, Li X, Tham YC, Liao J, Wong TY, Aung T, et al. Glaucoma in Asia: Regional prevalence variations and future projections. 100, Br J Ophthalmol. BMJ Publishing Group; 2016 [cited 2020 Aug.78–85. https://doi.org/10.1136/bjophthalmol-2014-306102
George R, Ve RS, Vijaya L. Glaucoma in India: Estimated burden of disease. J Glaucoma. 2020;19:391–7. https://pubmed.ncbi.nlm.nih.gov/20711029/
Quigley H, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol Br J Ophthalmol. 2020;90:262–7. https://pubmed.ncbi.nlm.nih.gov/16488940/
Vijaya L, George R, Arvind H, Baskaran M, Ve Ramesh S, Raju P, et al. Prevalence of Primary Angle-Closure Disease in an Urban South Indian Population and Comparison with a Rural Population. The Chennai Glaucoma Study. Ophthalmology. 2020;115:655–61. https://pubmed.ncbi.nlm.nih.gov/17869343/
He M, Friedman DS, Ge J, Huang W, Jin C, Lee PS, et al. Laser Peripheral Iridotomy in Primary Angle-Closure Suspects: Biometric and Gonioscopic Outcomes. The Liwan Eye Study. Ophthalmology. 2020;114:494–500. https://pubmed.ncbi.nlm.nih.gov/17123610/
Azuara-Blanco A, Burr J, Ramsay C, Cooper D, Foster PJ, Friedman DS, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet. 2020;388:1389–97. www.thelancet.com
Foster PJ, Johnson GJ. Glaucoma in china: How big is the problem? Br J Ophthalmol. 2020;85:1277–82. https://pubmed.ncbi.nlm.nih.gov/11673287/
Thomas R. Glaucoma in developing countries. Indian J Ophthalmol. 2012;60:446–50.
Thomas R, Sekhar GC, Parikh R. Primary angle closure glaucoma: A developing world perspective. Clin Exp Ophthalmol. 2020;35:374–8. https://pubmed.ncbi.nlm.nih.gov/17539793/
Lavanya R, Foster PJ, Sakata LM, Friedman DS, Kashiwagi K, Wong TY, et al. Screening for Narrow Angles in the Singapore Population: Evaluation of New Noncontact Screening Methods. Ophthalmology 2020;115. https://pubmed.ncbi.nlm.nih.gov/18486215/
Thomas R, George T, Braganza A, Muliyil J. The flashlight test and van Herick’ s test are poor predictors for occludable angles. Aust N. Z J Ophthalmol. 2020;24:251–6. https://pubmed.ncbi.nlm.nih.gov/8913128/
Johnson TV, Ramulu PY, Quigley HA, Singman EL. Low Sensitivity of the Van Herick Method for Detecting Gonioscopic Angle Closure Independent of Observer Expertise. Am J Ophthalmol. 2020;195:63–71. http://www.ncbi.nlm.nih.gov/pubmed/30071210
Chansangpetch S, Rojanapongpun P, Lin SC. Anterior Segment Imaging for Angle Closure. Am J Ophthalmol. 2020;188:xvi–xxix. http://www.ncbi.nlm.nih.gov/pubmed/29352976
Dorairaj S, Tsai JC, Grippo TM. Changing Trends of Imaging in Angle Closure Evaluation. ISRN Ophthalmol. 2012;2012:1–7. https://pubmed.ncbi.nlm.nih.gov/24558589/
Nongpiur ME, Khor CC, Cheng CY, Husain R, Boey PY, Chew A, et al. Integration of Genetic and Biometric Risk Factors for Detection of Primary Angle Closure Glaucoma. Am J Ophthalmol. 2019;208:160–5.
Nolan WP, Baasanhu J, Undraa A, Uranchimeg D, Ganzorig S, Johnson GJ. Screening for primary angle closure in Mongolia: A randomised controlled trial to determine whether screening and prophylactic treatment will reduce the incidence of primary angle closure glaucoma in an east Asian population. Br J Ophthalmol. 2020;87:271–4. https://pubmed.ncbi.nlm.nih.gov/12598435/
Markowitz SN, Donald, Morin J. The ratio of lens thickness to axial length for biometric standardization in angle-closure glaucoma. Am J Ophthalmol. 2020;99:400–2. https://pubmed.ncbi.nlm.nih.gov/3885747/
Niu WR, Dong CQ, Zhang X, Feng YF, Yuan F. Ocular biometric characteristics of Chinese with history of acute angle closure. J Ophthalmol. 2018;2018:1–6. 58357
Razeghinejad MR, Banifatemi M. Ocular biometry in angle closure. J Ophthalmic Vis Res. 2020;8:17–24. https://pubmed.ncbi.nlm.nih.gov/23825708/
Congdon NG, Quigley HA, Hung PT, Wang TH, Ho TC. Screening techniques for angle-closure glaucoma in rural Taiwan. Acta Ophthalmol Scand. 2020;74:113–9. https://pubmed.ncbi.nlm.nih.gov/8739673/. 1996.
Devereux JG, Foster PJ, Baasanhu J, Uranchimeg D, Lee PS, Erdenbeleig T, et al. Anterior chamber depth measurement as a screening tool for primary angle-closure glaucoma in an East Asian population. Arch Ophthalmol. 2020;118:257–63. https://pubmed.ncbi.nlm.nih.gov/10676792/
George R, Paul PG, Baskaran M, et al. Ocular biometry in occludable angles and angle closure glaucoma: A population based survey. Br J Ophthalmol. 2020:399–402. https://pubmed.ncbi.nlm.nih.gov/12642298/
Sihota R, Lakshmaiah NC, Agarwal HC, Pandey RM, Titiyal JS. Ocular parameters in the subgroups of angle closure glaucoma. Clin Exp Ophthalmol. 2000;28:253–8.
He M, Wang D, Zheng Y, Zhang J, Yin Q, Huang W, et al. Heritability of Anterior Chamber Depth as an Intermediate Phenotype of Angle-Closure in Chinese: The Guangzhou Twin Eye Study. Investig Ophthalmol Vis Sci. 2008;49:81–6. https://doi.org/10.1167/iovs.07-1052
Lee RY, Chon BH, Lin S-C, He M, Lin SC. Association of Ocular Conditions With Narrow Angles in Different Ethnicities. Am J Ophthalmol. 2020;160:506–515.e1. https://linkinghub.elsevier.com/retrieve/pii/S0002939415003414
Saxena S, Agrawal PK, Pratap VB, Nath R, Saxena RC. The predictive value of the relative lens position in primary angle-closure glaucoma. Ann Ophthalmol. 2020;25:453–6. http://www.ncbi.nlm.nih.gov/pubmed/8129328
Marchini G, Pagliarusco A, Toscano A, Tosi R, Brunelli C, Bonomi L. Ultrasound biomicroscopic and conventional ultrasonographic study of ocular dimensions in primary angle-closure glaucoma. Ophthalmology. 2020;105:2091–8. https://pubmed.ncbi.nlm.nih.gov/9818611/
Nongpiur ME, He M, Amerasinghe N, Friedman DS, Tay WT, Baskaran M, et al. Lens vault, thickness, and position in chinese subjects with angle closure. Ophthalmology. 2020;118:474–9. https://pubmed.ncbi.nlm.nih.gov/21035864/
Lowe RF. Aetiology of the anatomical basis for primary angle-closure glaucoma. Biometrical comparisons between normal eyes and eyes with primary angle-closure glaucoma. Br J Ophthalmol. 2020;54:161–9. https://pubmed.ncbi.nlm.nih.gov/5428641/
Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5.
Nolan WP, See JL, Chew PTKK, Friedman DS, Smith SD, Radhakrishnan S, et al. Detection of Primary Angle Closure Using Anterior Segment Optical Coherence Tomography in Asian Eyes. Ophthalmology. 2020;114:33–9. http://www.ncbi.nlm.nih.gov/pubmed/17070597
Aung T, Nolan WP, Machin D, Seah SKL, Baasanhu J, Khaw PT, et al. Anterior chamber depth and the risk of primary angle closure in 2 East Asian populations. Arch Ophthalmol Arch Ophthalmol. 2005;123:527–32. https://pubmed.ncbi.nlm.nih.gov/15824227/
Natarajan M, Das K, Jeganathan J. Comparison of central corneal thickness of primary open angle glaucoma patients with normal controls in South India. Oman J Ophthalmol. 2021;6:33. /pmc/articles/PMC3678195/
Olurin O. Anterior chamber depths of Nigerians. Ann Ophthalmol. 1977;9:315–26.
Chen Y-Y, Chen Y-Y, Sheu S-J, Chou P. The biometric study in different stages of primary angle-closure glaucoma. Eye (Lond). 2013;27:1070–6.
He M, Jiang Y, Huang S, Chang DS, Munoz B, Aung T, et al. Laser peripheral iridotomy for the prevention of angle closure: a single-centre, randomised controlled trial. Lancet (Lond, Engl). 2022;393:1609–18. https://pubmed.ncbi.nlm.nih.gov/30878226/
Baskaran M, Kumar RS, Friedman DS, Lu QS, Wong HT, Chew PTK, et al. The Singapore Asymptomatic Narrow Angles Laser Iridotomy Study: Five-Year Results of a Randomized Controlled Trial. Ophthalmology. 2022;129:147–58. http://www.aaojournal.org/article/S0161642021006357/fulltext
Thomas R, George R, Parikh R, Muliyil J, Jacob A. Five year risk of progression of primary angle closure suspects to primary angle closure: a population based study. Br J Ophthalmol. 2022;87:450–4. https://pubmed.ncbi.nlm.nih.gov/12642309/
Thomas R, Parikh R, Muliyil J, Kumar RS. Five-year risk of progression of primary angle closure to primary angle closure glaucoma: a population-based study. Acta Ophthalmol Scand. 2022;81:480–5. https://pubmed.ncbi.nlm.nih.gov/14510795/
Ye T, Yu Q, Peng S, Wang N, Chen X. [Six year follow-up of suspects of primary angle-closure glaucoma] - PubMed. Zhonghua Yan Ke Za Zhi. 2022;34:167–9. https://pubmed.ncbi.nlm.nih.gov/11877180/
International Diabetes Federation: India diabetes report 2000 — 2045. 2021 [cited 2022 Jan 27]. https://www.diabetesatlas.org/data/en/country/93/in.html
National Programme for Control of Blindness State wise targets & Achievement for various eye diseases during 2018-19. NPCBVI, Government of India -. 2018. https://npcbvi.gov.in/Home
Funding
SK is a recipient of the DBT/Wellcome Trust India Alliance fellowship grant. However, this research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SSav: was involved in conceptualising and designing the study, literature search, data collection, performing the tests, paper preparation, editing and reviewing. SK was involved in conceptualising and designing the study, literature search, data analysis & interpretation of results, paper preparation, editing and reviewing. SK will act as the guarantor of the paper. AD was involved in literature search, data acquisition and performing tests, paper editing and reviewing. SS and DK: were involved in data analysis, paper editing and reviewing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Savur, S., Kaup, S., Dinesh, A. et al. Can ultrasonic biometric indices with optimal cut-offs be a potential screening tool for primary angle closure disease? A case-control study. Eye 37, 1284–1289 (2023). https://doi.org/10.1038/s41433-022-02118-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02118-y