NCCN Recommends Full COVID-19 Vaccination ASAP for Most Patients With Cancer
Patients with cancer should be fully immunized against COVID-19, including third doses and/or any approved boosters.
Robert W. Carlson, MD

Patients with cancer should be fully immunized against COVID-19, including third doses and/or any approved boosters, according to the most recent recommendations published by the National Comprehensive Cancer Network (NCCN) Advisory Committee on COVID-19 Vaccination and Pre-exposure Prophylaxis.
“The medical and scientific community’s response to the COVID-19 crisis continues to be extremely encouraging, even in the face of setbacks [such as] new variants and surging infection rates,” said Robert W. Carlson, MD, CEO of the NCCN, in a press release. “Rapid research, thoughtful analyses, and tireless care delivery is allowing us to save so many more people than we could have a year ago. We hope by sharing this simplified guidance highlighting the latest research and approvals, we can help make sure the very latest in evidence-based care reaches as many patients and providers as possible.”
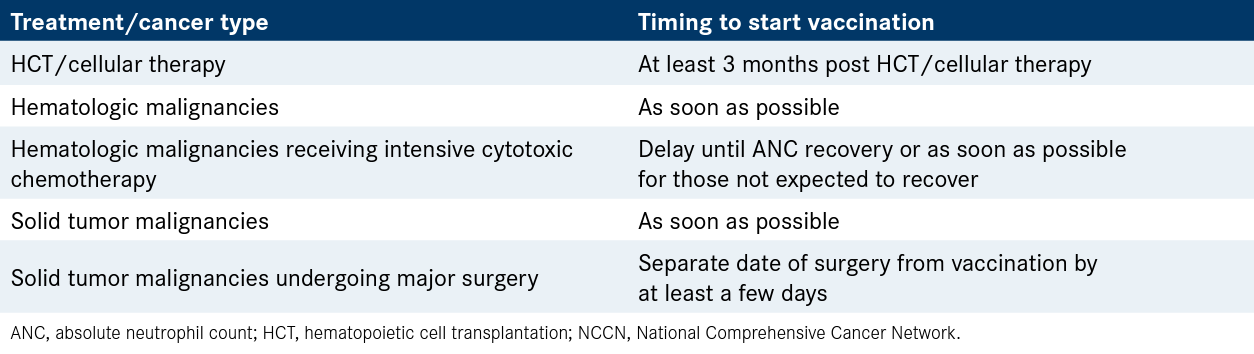
Table. General NCCN COVID-19 Vaccination Recommendations for Patients With Cancer
The committee expressed a strong preference for mRNA vaccines for patients with cancer vs adenovirus vector vaccines. The Pfizer/BioNTech (BNT162b2) and Moderna (mRNA1273) vaccines are both mRNA vaccines given in a 2-dose series. The Janssen/Johnson & Johnson (Ad26.COV2.S) is an adenovirus vector vaccine given as a single dose.
Patients with solid tumor malignancies should be fully vaccinated as soon as possible, with the exception of those undergoing major surgery. Major surgery patients should separate the date of surgery from vaccination by at least a few days.
For those patients with hematologic malignancies receiving intensive cytotoxic chemotherapy, vaccination should be delayed until absolute neutrophil count (ANC) recovery. Patients who are not expected to achieve ANC recovery and all other patients with hematologic malignancies should be vaccinated as soon as possible.
Patients undergoing hematopoietic cell trans-plantation (HCT) and/or cellular therapy should wait at least 3 months after the conclusion of their therapy to be vaccinated. This includes patients being treated with allogeneic transplantation, autologous transplantation, and chimeric antigen receptor (CAR) T-cell therapy.
In terms of a third dose of the mRNA vaccines, the NCCN committee supports the additional dose for patients with cancer who are immunocompro-mised, including those who are receiving active cancer treatment for solid tumors or cancers of the blood. For patients who received the adeno-virus vector vaccine, a second dose of the same vaccine or of the mRNA vaccine should be given in place of the third dose.
A third dose is recommended for patients with solid tumor malignancies who have received cancer treatment within 1-year of initial vaccine administration. Patients with hematologic malignancies should receive an additional dose regardless of whether or not they are receiving cancer therapy, due to the fact that they are at high risk for poor serologic responses to vaccination both as a result of immunodeficiency due to the malignancy and their associated cancer therapies. The committee added that patients who have undergone treatment with HCT and/or cellular therapy should receive the third dose, with priority given to those who are less than 2 years post procedure.
The committee noted that it is important to understand the difference between a third dose for immunocompromised patients and the booster dose given to the general public. The third full dose of the mRNA vaccines is 100 μg and 30 μg for the mRNA-1273 and BNT162b2 vaccines, respectively. The dose of the booster vaccine is 50 μg for mRNA-1273 and 30 μg for BNT162b2. Among patients with a history of cancer, those who do not meet the criteria for the third vaccine dose given to immunocompromised patients should be offered a booster similar to the general population at 6 months after completion of their primary vaccine series.
Regarding the timing of the third dose, the committee agrees with the CDC that the additional dose of the mRNA vaccine should be administered at least 4 weeks after the second dose, preferably with the same vaccine that was used for the first 2 doses. The NCCN recommends a second dose for those who received the Janssen/Johnson & Johnson vaccine 2 months after the first dose with preference given to an mRNA vaccine vs a Janssen/Johnson & Johnson second dose. For patients who initially received the Janssen/Johnson & Johnson vaccine, 2 additional doses, at least 28 days apart, are recommended by the NCCN for high-risk patients and a booster at 6 months following the third dose.
When selecting patients to receive a third dose and an additional booster, the decision should be made based on the underlying cancer, therapy, and other immunocompromising conditions. The committee does not recommend the use of antibody titers to determine if patients should receive additional doses of vaccine, outside of a research study context. Patients with cancer who have a history of COVID-19 following their initial vaccine series should also receive a third dose, delayed at least 29 days post completed vaccine series and documented clearance of SARSCoV-2 virus.
For patients who may not mount an adequate immune response to the vaccine series, the NCCN endorses the monoclonal antibody combination of tixagevimab plus cilgavimab for pre-exposure protection from COVID-19. To avoid interference with vaccine-induced immunity, tixagevimab plus cilgavimab should be administered at least 2 weeks after COVID-19 vaccination. Because of the limited supply of the combination, the committee concluded that cancer centers should prioritize distribution to patients with hematologic malignancies as these patients are more likely to have an inadequate immune response to vaccination.
Reference
- NCCN. Cancer and COVID-19 vaccination, version 5.0. January 4, 2022. Accessed January 6, 2022.bit.ly/3zQcf8T




