Abstract
Background
Childhood asthma is an inflammatory disease with heterogeneous outcomes. We sought to determine the impact of total IgE, blood eosinophil, allergen sensitization, and inhaled corticosteroid (ICS) on longitudinal outcomes and to identify characteristics for discriminating different outcomes.
Methods
We conducted a retrospective study in 383 childhood asthma patients and another 313 patients with blood eosinophil data only receiving regular program-based visits from September 1, 2004, to December 31, 2018. Peak expiratory flow (PEF) variability, PEF predicted %, asthma severity, and asthma control at each visit were assessed as clinical outcomes.
Results
Our data show that the percentage of blood eosinophils was significantly associated with increased asthma severity (OR: 1.043, 95% CI: 1.002–1.086, P = 0.0392). Mold sensitization was significantly associated with asthma severity (OR: 2.2485, 95% CI: 1.3253–3.8150, P = 0.0027). Characteristics including sensitization status plus ICS dosage had the best area under the receiver operating characteristic curve (AUC) value for predicting longitudinal PEF predicted % (0.6609), PEF variability (0.6885), asthma severity (0.5918), and asthma control (0.6441), respectively.
Conclusions
We showed that the risk for adverse clinical outcomes at follow-up differed between serum IgE, blood eosinophil, and allergen sensitization identified at baseline. Sensitization and ICS dosage were predictive characteristics of long-term clinical outcomes.
Impact
-
The unique aspects of the study are its longitudinal assessment of patients receiving guideline-based asthma management program to help characterize the stability of the clinical outcomes over time. Characteristics including allergen sensitization and ICS dosage demonstrated an improved capability for distinguishing between better and worse clinical outcomes. Through longitudinal serial assessment, this study indicates the risk for adverse clinical outcomes differed between children with serum IgE/blood eosinophil/allergen sensitization characterized at baseline.
Similar content being viewed by others
Introduction
Childhood asthma is a heterogeneous disease with diverse clinical and molecular phenotypes1. The major focus of asthma investigations is on the underlying immunoglobulin E (IgE)-mediated inflammatory mechanisms. T-helper 2 (Th2)-high asthma is characterized by exaggerated airway hyperresponsiveness, high total serum IgE, and peripheral blood eosinophilia2. Total serum IgE is related to the severity of atopy in allergic diseases3. In children, however, there are conflicting results regarding the relationship of asthma severity and control with total serum IgE. Some works show that the total serum IgE determines atopy but not its severity4, whereas others report a positive correlation between total serum IgE and asthma severity and control in children5,6,7,8,9,10,11,12.
Serial measurements of blood eosinophil count correlate with asthma severity, and can be used for monitoring changes therein13,14,15. Blood eosinophilia is also observed in pediatric asthma and is related to disease severity16. Blood eosinophil counts have traditionally been used to monitor asthma control status17. There is a direct relationship between eosinophil count and the frequency of asthma exacerbations (that is, poor asthma control)18. Few studies of the association of both asthma severity and control with peripheral blood eosinophils have involved pediatric patients.
Studies attempting to identify childhood asthma phenotype outcomes are hampered by the fact that most studies characterized patients at a single time point19. Furthermore, it is not clear whether controller therapy has been optimized based on guidelines and that compliance with treatment has been carefully assessed11. Furthermore, it is very challenging to demonstrate airflow limitation using the available lung function tests (i.e., spirometry, bronchodilation test, bronchial challenges), especially in children20. Most children with persistent asthma exhibit abnormal pulmonary function only during acute exacerbations. Characterization of asthma severity and control in pediatric patients should consider these observations.
The reported variables used by the area under the receiver operating characteristic curve (AUROC) method to predict asthma include sputum eosinophil21, blood eosinophil22, biomarker such as serum eosinophil-derived neurotoxin17 and fractional exhaled nitric oxide (FeNO) levels, pulmonary function23, childhood asthma control test (c-ACT)24, and other clinical information. Incorporating information of both inhaled corticosteroid (ICS) and allergen sensitization to improve the AUC for predicting asthma risk had also been recently reported25. There are insufficient data supporting the use of baseline characteristics (total serum IgE level, percentage of blood eosinophil, allergen sensitization) along with follow-up ICS dosage using the AUROC method to improve discrimination of specific outcomes of asthma (e.g., asthma severity and asthma control).
The objective of this analysis was to assess the impact of total IgE, blood eosinophil, allergen sensitization, and controller ICS dosage on the longitudinal asthma outcomes, including lung function measured with peak expiratory flow (PEF), asthma severity, and asthma control, in a population of childhood asthma receiving asthma management program. We also aimed to evaluate the discriminative potential of these characteristics alone or in combination in discriminating the long-term outcome of childhood asthma.
Methods
Improvement program for health care benefits of asthma (National Health Insurance, Taiwan)
In September 2004, National Taiwan University Hospital joined this program. The program introduces incentives to improve the quality of asthma care, encourages hospitals to engage in patient-centered complete asthma care, improves compliance with guidelines, strengthens follow-up visits and health-education services for asthmatic patients, and ensures the provision of complete and continuous care. This program also uses asthma case managers to provide a multifaceted approach to asthma care for “high-risk” outpatients; this approach is tailored to patients and is implemented every 3 months (refer to Supplementary Material for detail of program component).
Inclusion criteria
The inclusion criteria for patients were as follows: diagnosed with asthma within 90 days prior to enrollment by the same physician and had completed at least two outpatient visits to that physician; willing to comply with asthma care regulations and with follow-up contacts by our asthma case managers; and received care and follow-up from their physician for at least 3 months.
End of enrollment
1. Completion of self-care and self-evaluation (disease resolved).
2. Refusal by the patient of further contact by asthma case managers and/or follow-up by asthma specialists.
3. Loss of contact and/or follow-up for >90 days.
4. Change in place of residence.
5. Death.
6. Noncompliance with the program regulations for >1 year.
7. Others (e.g., transfer to other care providers, change in diagnosis).
These patients continued to receive medications and treatments for their asthma as indicated by their asthma specialist and the Global Initiative for Asthma (GINA) guidelines26. The data were collected at baseline (total serum IgE and blood eosinophil percentage), and the following clinical data were collected by asthma case managers during interviews and follow-up visits: (1) tracking/visit date, (2) frequency of daytime symptoms, (3) frequency of nighttime symptoms, (4) peak expiratory flow (PEF) (predicted %), (5) PEF variability (%), (6) asthma severity, and (7) asthma control.
Enrolled patients were provided comprehensive information on asthma care, including the pathophysiology of asthma, how to diagnose asthma, asthma severity, environmental allergens, and control, management of acute attacks, long-term control of asthma, asthma relievers and controllers, and the importance of monitoring PEF. We instructed the patients in the correct method of inhalation, the timing of medications, and measurement of the peak PEF at home.
Patients
Asthma diagnosis
Asthma diagnoses were made based on the following criteria: history of cough, recurrent wheezing, recurrent difficulty breathing, and recurrent chest tightness. Symptoms tend to occur or worsen at night or with exercise, viral infection, exposure to allergens and irritants, changes in weather, hard laughing or crying, and stress. Diagnostic criteria also included a family history of eczema, hay fever, asthma, or atopic disease. Complete physical examinations focused on both the upper and lower respiratory tracts, chest (use of accessory muscles, appearance of hunched shoulders, barrel configuration to the chest indicating chronic air trapping), nose (allergic “shiners,” allergic crease), and skin (atopic dermatitis). Radiographic studies may indicate radiographic signs of air trapping, such as hyperlucency, flattening of the diaphragms, increased anteroposterior (AP) diameter, or horizontal positioning of the ribs.
Children 5 years and younger with clinical features and characteristics suggesting asthma and children 6–11 years and adolescents (12–18 years) meeting the diagnostic criteria were diagnosed with asthma according to the National Asthma Education and Prevention Program: Expert Panel Report 3 (EPR-3)27 and Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA)26. From September 1, 2004, to December 31, 2018, we registered 1115 eligible children receiving regular program-based visits. Among them, 383 children with data on total serum IgE, allergen sensitization and percentage of blood eosinophil were enrolled. Another 313 children with percentages of blood eosinophil data only were also enrolled as the validation cohort. Data on PEF variability, PEF predicted %, asthma severity, and asthma control during the follow-up of these 696 patients were collected as clinical outcomes according to EPR-327. Asthma and rhinitis were optimally managed by study physicians according to the applicable guidelines. This study was approved by the Institutional Review Board and Research Ethics Committee of the National Taiwan University Hospital and was conducted in compliance with the protocol for good clinical practices and the principles of the Declaration of Helsinki. The informed consents were obtained from all participants and/or their legal guardians.
Descriptive statistics were calculated for demographic and baseline characteristics. IgE values in excess of 5000 IU/mL were assigned a value of 5000 IU/mL. The geometric mean (GM) and the arithmetic mean (AM) were calculated to approximate the normal distribution for statistical inference and modeling.
Peak expiratory flow (PEF)
We measured the PEF variability and predicted the PEF percentage according to the Guidelines for the Diagnosis and Management of Asthma (EPR-3)27. Diurnal PEF variability is calculated from twice daily PEF measurements as follows: ([day’s highest minus day’s lowest]/mean of day’s highest and lowest), averaged over 1 week. Good reproducible results for peak expiratory flow rates could be obtained in children under 5 years of age28.
Sensitization
Allergen sensitization was determined with multiple allergen simultaneous tests: MAST Optigen which contain 36 kinds of allergens (Hitachi Chemical Diagnostics, Mountain View, CA). Sensitization results were interpreted as class 0–4 with MAST Optigen luminometer. Class >1 was interpreted as positive sensitization.
Thirty-six serum allergen-specific IgEs (sIgEs) were grouped into the following categories: dust mites (Pyroglyphidae, Dermatophagoides farinae, Dermatophagoides pteronyssinus), danders/feathers (chicken feather: Gallus gallus; cat: Felis domesticus; dog: Canis familiaris), molds (Alternaria alternata, Aspergilus fumigatus, Cladosporium herbarum, Penicillium notatum), grasses/trees (Bermuda grass: Cynodon dactylon; Willow black: Salix nigra; Eucalyptus: Eucalyptus globulus; Cedar Japan: Crytomeria japonica; mulberry mix: Morus alba; pigweed mix: Amaranthus retroflexus; ragweed mix: Ambrosia Elatior+ Ambrosia Trifida; Timothy grass: Phleum pratense), foods (avocado: Persea Americana; pork: Sus scrofa; beef: Bos taurus spp.; milk, cheddar cheese; shrimp: Penaeus spp.; crab: Callinectes sapidus; clam: Mercenaria mercenaria; codfish: Gadus morhua; tuna: Thunnus sp.; peanut: Arachis hypogaea; soybean: Glycine max; wheat: Triticum aestivum; brewer’s yeast, egg yolk, egg white), cockroach mix (Blattella Germanica+ Periplaneta Americana), and latex (Hevea brasiliensis).
Statistical analysis
Generalized estimating equations (GEEs) are important in the analysis of correlated data29. These data sets can be generated in longitudinal studies, in which patients are measured at different points in time, or in cluster studies, in which measurements are taken of patients who share a common characteristic. We performed GEE analysis using the GENMOD and GEE procedures in SAS software. Missing data are common in longitudinal studies and can be caused by dropouts or skipped visits. Both procedures implement the standard GEE approach for longitudinal data; this approach is appropriate for complete data or when data are missing completely at random.
We first analyzed the association between independent variables (characteristics: total serum IgE, percentage of blood eosinophil, allergen sensitization, and follow-up ICS dosage) and dependent variables (clinical outcomes). We conducted this analysis using the 3-level ICS dose classification. Low, medium, and high daily doses of inhaled corticosteroids (ICS) of estimated comparability were defined by GINA26. The patients were stratified by asthma severity for further analysis. Patients with the severity of either mild intermittent or mild persistent were divided into low severity groups. Patients with the severity of either moderate persistent or severe persistent were divided into high severity groups. The odds ratio (OR) presented herein are calculated via GEE analysis using the binary severity indicator as to the outcome variable (Table 1). Significant univariate variables (P < 0.05) were included for multivariable analysis.
Area under a receiver operating characteristic (AUROC) curve analysis
AUROC analysis was applied to differentiate children who would be a worse outcome from those who would be a better outcome. Four independent variables (total serum IgE, blood eosinophil %, allergen sensitization, and ICS dosage) will be used to predict 4 outcomes (PEF predicted %, PEF variability, asthma severity, asthma control). Logistic regression models were used to compare the predictive performance of the combinations of independent variables.
Results
Patients’ characteristics
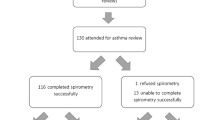
The mean follow-up times of our 383 patients were 16.4 times (range: 3–97 times). The median follow-up times were 12 times (interquartile range: 6–24 times) (Fig. 1). We had performed sensitivity analysis by re-analyzing the OR results again but with each time dropping the data from patients with a lower number of follow-ups (below 5 times), dropping the data from patients with higher number of follow-ups (above 40 times), and dropping the data from patients with either below 5 times or above 40 times, respectively (Supplementary Fig. 1). Generally speaking, the results were similar when sensitivity analysis was applied directly to re-analyze the data with dropping a different set of follow-up times (<5 times, <5 or >40 times, and >40 times) compared with the original results before the sensitivity test.
a Flow diagram illustrating study subject selection, clinic follow-up visits, baseline data, inhaled corticosteroid dose, and clinical outcomes collected throughout the study. b Pareto chart plot of clinic follow-up visits of 383 patients. The orange line showed the accumulated percentages of patients who received different times of clinic follow-up visits. The numbers below the columns indicate the range of times of outpatient visits for participants within that column. For example, [0,5] means there were 83 patients who received 0–5 times clinic follow-up visits while [95,100] means there was 1 patient who received 95–100 times clinic follow-up visits.
The demographic characteristics of the 383 patients are presented in Table 2. The most common degree of asthma severity was intermittent to mild persistent severity (79.38%). The most frequent level of asthma control was partially well controlled (55.65%). Most patients were sensitized to mites (78.59%) and concurrently suffered from AR (53.0%).
Relationship between total serum IgE levels and percentage of blood eosinophils with allergen sensitization and comorbidity
Pearson’s correlation analysis revealed a moderate correlation between total serum IgE level and percentage of blood eosinophils (Pearson’s r = 0.3473, P < 0.0001) (Fig. 2). The sensitizations to all allergens were significantly correlated with total serum IgE. In univariate analyses, the percentage of blood eosinophil was associated with mite and dander/feather sensitization. The total serum IgE level, but not the percentage of blood eosinophils, was associated with AR and/or AD (Supplementary Table 1).
Effect of baseline total serum IgE level and percentage of blood eosinophil on longitudinal asthma outcomes
Total serum IgE levels were transformed into a log scale and divided by 1,000, respectively, and considered a continuous variable for the statistical models. The GEE analysis showed that IgE level (divided by 1,000) was slightly but not significantly associated with increased PEF variability (OR 1.3354, 95% CI: 0.8862–2.0124, P = 0.1668). Log IgE tended to be associated with better asthma control, but the association was not significant (OR = 0.8546; 95% CI: 0.7023–1.0398; P = 0.1164) (Fig. 3a, b). Of the 383 patients, percentage of blood eosinophils was marginally associated with asthma severity and with asthma control (OR: 1.0522, 95% CI: 0.9976–1.1098, P = 0.0614; OR: 1.0392, 95% CI: 0.9987–1.0814, P = 0.0579; respectively). We analyzed the validation cohort of another 313 patients with eosinophils data only (Supplementary Table 2) and found that elevated percentage of blood eosinophils tended to be associated with increased asthma severity and with poor asthma control (OR: 1.0444, 95% CI: 1.0033–1.0871, P = 0.034; OR: 1.039, 95% CI: 0.9762–1.1057, P = 0.229; respectively) (Fig. 3a, b). When the data from two groups of patients were pooled together (total 696 patients), the percentage of blood eosinophils was significantly associated with increased asthma severity (OR: 1.043, 95% CI: 1.002–1.086, P = 0.0392). For patients with higher asthma severity, the percentage of blood eosinophils was significantly associated with worse asthma control (OR: 1.127, 95% CI: 1.010–1.257, P = 0.0323) (supplement Fig. 2).
Effect of total serum IgE and blood eosinophil (a, b), allergen sensitization (c, d), ICS dosage (e, f) and co-morbidities (AR, AD) (g, h) on OR (95% CI) of having better or worse asthma outcome: PEF predicted % (>80% or 60–80% versus <60%), PEF variability (<20% versus 20–30% or >30%), asthma severity (mild intermittent or mild persistent versus moderate persistent or severe persistent), and asthma control (well-controlled or partially controlled versus poorly controlled). Legend: Eosinophil (develop): blood eosinophil % data from 383 patients of develop cohort; Eosinophil (validate): blood eosinophil % data from 313 patients of validate cohort; eosinophil % (pooled): blood eosinophil % data from 383 patients plus 313 patients (total 696 patients).
Longitudinal associations between baseline allergen sensitization and asthma outcomes
Mold sensitization was significantly associated with asthma severity (OR: 2.2573, 95% CI: 1.3305–3.8299, P = 0.0025) and was validated in a multivariate analysis (OR: 2.2485, 95% CI: 1.3253–3.8150, P = 0.0027) (Fig. 3c, d and Supplementary Table 3). Our findings were in consistent with that fungal sensitization is associated with disease severity in persistent childhood asthma11. Grass/trees sensitization tended to be positively associated with PEF variability, although the association was not significant (OR: 2.0407, 95% CI: 0.9004–4.625, P = 0.0875). Patients with mite sensitization seemed likely to be associated with lower PEF variability (OR: 0.5199, 95% CI: 0.2918–0.9262, P = 0.0264) and with better asthma control (OR: 0.5801, 95% CI: 0.3787–0.8887, P = 0.0123). However, these associations were not significant in the multivariate analysis (Supplementary Table 3). These associations were also not significant when patients were stratified by asthma severity (Supplementary Fig. 2).
Longitudinal associations between follow-up ICS dosage and asthma outcomes
Medium-dose ICS (OR: 2.3419, 95% CI: 1.0388–5.2795, P = 0.0402) and high-dose ICS (OR: 4.5577, 95% CI: 1.1679–17.7859, P = 0.0290) were significantly associated with PEF variability. For patients with high asthma severity, high-dose ICS but not medium-dose ICS was positively associated with increased PEF variability (OR: 8.000, 95% CI: 1.452–44.064, P = 0.0169) (Supplementary Fig. 2). Medium-dose ICS was significantly associated with poor asthma control (OR: 2.0507, 95% CI: 1.1385–3.6939, P = 0.0168) (Fig. 3e, f). The association with almost all clinical outcomes followed a dose-response relationship; the odds of association with four outcomes increased progressively with ascending categories of ICS dosage compared with the reference category of low dose ICS. ICS use could be considered a marker of asthma severity30. The risk of asthma persistence was significantly increased among those with the use of inhaled steroids31. Our findings suggest that different asthma outcomes could be differentiated according to the longitudinal maintenance dose of ICS.
Area under the receiver operating characteristic (ROC) curve (AUROC)
We calculated prediction performance for the models based on total serum IgE alone, blood eosinophil percentage alone, allergen sensitization alone, and ICS dosage alone and successively combined with other variables to differentiate between better and worse clinical outcomes (Table 1). The analysis showed that, for all four types of outcomes, more variables added can improve the discriminative capacity. Overall, the best performance is the prediction of PEF variability, and the worst performance is the prediction of asthma severity (Fig. 4). The discriminative abilities of IgE + eosinophil + sensitization + ICS as variables for predicting PEF predicted % were with an AUC value of 0.6609. The best AUC for predicting PEF variability with sensitization + ICS as variables were 0.6885. The combination of sensitization + ICS had a better predictive effect on asthma severity with the best AUC of 0.5918. The ROC curve indicated that IgE + sensitization + ICS had the highest predictive accuracy for asthma severity (AUC = 0.6441). Except for asthma severity, our AUC value is with acceptable discrimination (0.7 ≥ AUC > 0.6)32. We demonstrated that characteristics including allergen sensitization plus ICS dosage demonstrated a predictive capability for distinguishing between better and worse clinical outcomes.
Comparison of prediction performance of area under receiver operating characteristic (ROC) curves for discrimination of specific asthma outcomes of a PEF predicted %, b PEF variability, c asthma severity, and d asthma control with different models using total serum IgE, blood eosinophil percentage, allergen sensitization, and ICS dose as univariate variables in 383 children with asthma. Compared with total serum IgE or blood eosinophil percentage, the combination of allergen sensitization plus ICS dosage were better predictors of all outcome status. Overall, the best performance is the prediction of PEF variability (AUC ≅ 0.65), and the worst performance is the prediction of asthma severity (AUC ≅ 0.55). The dotted line at 0.5 corresponds to the AUC value where a prediction model discriminates worse outcome from better outcome not better than at random.
Discussion
We showed that the risk for adverse clinical outcomes at follow-up differed between serum IgE, blood eosinophil, and allergen sensitization identified at baseline. Most childhood asthma studies support the finding that total IgE is positively correlated with asthma severity (Supplementary Table 4). High total IgE has been reported to be associated with uncontrolled asthma11,30. Our data show that total serum IgE levels tended to be associated with increased PEF variability. Individual peak flow measurements are highly variable, and the PEF variability has greater predictive power for future exacerbations than individual PEF measurements33. Blood eosinophil counts have traditionally been used to monitor asthma control17. We demonstrated that the percentage of blood eosinophils was associated with long-term both asthma severity and asthma control in different patient cohorts. Our data on blood eosinophils is useful for the management of childhood asthma.
The GINA guidelines and multiple clinical trials have helped to guide current evidence-based treatments and to maximize the overall level of asthma control26. However, it is not possible to change the natural history of the disease. Despite therapy to control asthma symptoms and acute exacerbations, the outcomes of asthma appear largely resistant to pharmacological therapy34,35. Notably, the clinical pattern of greater asthma severity occurred despite sustained use of the highest step levels of guideline-based asthma controllers for years11. Because our study focused on long-term asthma disease course and there were a comparable number of patients in our study, the fact that ICS did not change the asthma phenotype outcome might be applicable to our study. Because there was a dose-response relationship between ICS dose and all asthma outcomes, thus, we suggest that ICS dose at follow-up can be used as a good parameter for predicting long-term asthma outcomes.
PEF is a valuable, easily available measurement that is well suited for monitoring trends in asthma control over time27. PEF determinations help document the variable nature of a patient’s airflow limitation (obstruction), which is a central feature of asthma36. Increases in the sputum eosinophil count are correlated with decreases in morning PEF and loss of asthma control. Several studies have demonstrated that blood eosinophil counts correlate well with asthma severity in children14. We showed that the percentage of blood eosinophil was associated with higher asthma severity and poorer asthma control. We considered blood eosinophilia is a consequence of systemic inflammation and reflects the degree of airflow limitation37,38. Here, we suggest PEF as an alternative objective measurement of airflow status in children with asthma.
A considerable problem with studies attempting to identify asthma phenotype outcomes is that patient characterization occurs as a single evaluation and does not include a measure of outcome stability over time11. Furthermore, in most of these studies, it is unclear whether therapy has been optimized based on guidelines and whether treatment adherence has been carefully assessed. Boudier et al. reported that most studies have relied on short to medium follow‐ups and suggested strong tracking of asthma activity and lung function over time39. Howrylak et al. showed that differences in clinical characteristics observed between children assigned to different clusters persist into young adulthood9. We suggest that serial longitudinal asthma outcomes assessments can be used as an observer-independent and data-driven asthma methodology that corresponds to the clinical phenotypes of childhood asthma.
Most research on predicting asthma has focused on predicting the diagnosis of asthma, such as with the Asthma Predictive Index (API). Our prediction tool can be used to identify ways to discriminate better from worse asthma severity, asthma control, and pulmonary function changes (PEF). In particular, the AUC from models integrating allergen sensitization and ICS dosage improved the discriminative capacity25. A specific IgE sensitization is demonstrable in a significant proportion of patients with severe forms of asthma12. We used total serum IgE, percentage of blood eosinophils, allergen sensitization, and ICS dosage as our predictors. In addition to the OR as a correlation indicator, our research further identified predictive methods (AUROC). Our method will help with clinical applications to predict asthma severity, asthma control, and changes in lung function (PEF).
There are some limitations. The effects of ICS treatment of longitudinal cohort studies are difficult to ascertain, given that treatment is more likely to be offered to those with more severe disease (confounding by severity). Not the collection of variables in a broad range of domains. Genetic information had also been shown to modulate the effects of therapy and therefore might influence long-term outcomes.
Conclusion
Through long-term assessment, we demonstrated some baseline characteristics (blood eosinophil and allergen sensitization) were related to the subsequent manifestation of asthma outcomes. Given the paucity of data on the longitudinal impact of blood eosinophils on asthma outcomes and the emergence of new therapies (anti-IL-5 mAb), our data on blood eosinophils is potentially valuable. The unique aspects of the study are its longitudinal assessment of patients receiving guideline-based asthma management programs to help characterize the stability of the clinical outcomes over time. Characteristics including allergen sensitization and ICS dosage demonstrated an improved capability for distinguishing between better and worse clinical outcomes.
References
Wenzel, S. E. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat. Med. 18, 716–725 (2012).
Woodruff, P. G. et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am. J. Respir. Crit. Care Med. 180, 388–395 (2009).
Ahlstedt, S. & Murray, C. S. In vitro diagnosis of allergy: how to interpret IgE antibody results in clinical practice. Prim. Care Respir. J. 15, 228–236 (2006).
Siroux, V. et al. Relationships of allergic sensitization, total immunoglobulin E and blood eosinophils to asthma severity in children of the EGEA study. Clin. Exp. Allergy 33, 746–751 (2003).
Kovac, K., Dodig, S., Tjesic-Drinkovic, D. & Raos, M. Correlation between asthma severity and serum IgE in asthmatic children sensitized to dermatophagoides pteronyssinus. Arch. Med. Res. 38, 99–105 (2007).
Borish, L. et al. Total serum IgE levels in a large cohort of patients with severe or difficult-to-treat asthma. Ann. Allergy Asthma Immunol. 95, 247–253 (2005).
Carroll, W. D. et al. Asthma severity and atopy: how clear is the relationship? Arch. Dis. Child. 91, 405–409 (2006).
Fitzpatrick, A. M. et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J. Allergy Clin. Immunol. 127, 382–389.e381-313 (2011).
Howrylak, J. A. et al. Classification of childhood asthma phenotypes and long-term clinical responses to inhaled anti-inflammatory medications. J. Allergy Clin. Immunol. 133, 1289–1300.e1281-1212 (2014).
Naqvi, M. et al. Association between IgE levels and asthma severity among African American, Mexican, and Puerto Rican Patients with Asthma. J. Allergy Clin. Immunol. 120, 137–143 (2007).
Pongracic, J. A. et al. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J. Allergy Clin. Immunol. 138, 1030–1041 (2016).
Zoratti, E. M. et al. Asthma phenotypes in inner-city children. J. Allergy Clin. Immunol. 138, 1016–1029 (2016).
Kamfar, H. Z., Koshak, E. E. & Milaat, W. A. Is there a role for automated eosinophil count in asthma severity assessment? J. Asthma 36, 153–158 (1999).
Casciano, J. et al. Value of peripheral blood eosinophil markers to predict severity of asthma. BMC Pulm. Med. 16, 109 (2016).
Jatakanon, A., Lim, S. & Barnes, P. J. Changes in sputum eosinophils predict loss of asthma control. Am. J. Respir. Crit. Care Med. 161, 64–72 (2000).
Ulrik, C. S. Peripheral eosinophil counts as a marker of disease activity in intrinsic and extrinsic asthma. Clin. Exp. Allergy 25, 820–827 (1995).
An, J. et al. Serum eosinophil-derived neurotoxin better reflect asthma control status than blood eosinophil counts. J. Allergy Clin. Immunol. Pract. 8, 2681–2688.e2681 (2020).
Price, D. B. et al. Blood eosinophil count and prospective annual asthma disease burden: A UK Cohort Study. Lancet Respir. Med. 3, 849–858 (2015).
Lang, A. et al. Asthma severity in childhood, untangling clinical phenotypes. Pediatr. Allergy Immunol. 21, 945–953 (2010).
Badar, A. et al. Association between feno, total blood IgE, peripheral blood eosinophil and inflammatory cytokines in partly controlled asthma. J. Asthma Allergy 13, 533–543 (2020).
Deykin, A. et al. Sputum eosinophil counts predict asthma control after discontinuation of inhaled corticosteroids. J. Allergy Clin. Immunol. 115, 720–727 (2005).
Demarche, S. et al. Step-down of inhaled corticosteroids in non-eosinophilic asthma: a prospective trial in real life. Clin. Exp. Allergy 48, 525–535 (2018).
Alvarez-Gutiérrez, F. J. et al. Comparison of the asthma control test (Act) with lung function, levels of exhaled nitric oxide and control according to the global initiative for asthma (Gina). Arch. Bronconeumol. 46, 370–377 (2010).
Sommanus, S., Direkwattanachai, C., Lawpoolsri, S. & Sitcharungsi, R. Accuracy of childhood asthma control test among Thai childhood asthma patients. Asian Pac. J. Allergy Immunol. 36, 152–158 (2018).
Kreißl, S. et al. Reduced exhaled breath condensate pH and severity of allergic sensitization predict school age asthma. J. Allergy Clin. Immunol. Pract. 9, 1570–1577 (2021).
Hogan, A. D. & Bernstein, J. A. Gina updated 2019: landmark changes recommended for asthma management. Ann. Allergy Asthma Immunol. 124, 311–313 (2020).
Expert Panel Report 3 (Epr-3). Guidelines for the diagnosis and management of asthma-summary report 2007. J. Allergy Clin. Immunol. 120, S94–S138 (2007).
Milner, A. D. & Ingram, D. Peak expiratory flow rates in children under 5 years of age. Arch. Dis. Child. 45, 780–782 (1970).
Liang, K.-Y. & Zeger, S. L. Longitudinal data analysis using generalized linear models. Biometrika 73, 13–22 (1986).
Siroux, V. et al. Phenotypic determinants of uncontrolled asthma. J. Allergy Clin. Immunol. 124, 681–687.e683 (2009).
Neuman, Å. et al. Infant wheeze, comorbidities and school age asthma. Pediatr. Allergy Immunol. 25, 380–386 (2014).
Yang, S. & Berdine, G. The receiver operating characteristic (Roc) curve. Southw. Respir. Crit. Care Chron. 5, 34–36 (2017).
Lugogo, N., Que, L. G., Gilstrap, D. L. & Kraft, M. Murray and Nadel’s Textbook of Respiratory Medicine 731–750.e737 (Elsevier, 2016).
Sears, M. R. Predicting asthma outcomes. J. Allergy Clin. Immunol. 136, 829–836 (2015).
Lee-Sarwar, K. A., Bacharier, L. B. & Litonjua, A. A. Strategies to alter the natural history of childhood asthma. Curr. Opin. Allergy Clin. Immunol. 17, 139–145 (2017).
Irvin, C. G. Middleton’s Allergy 700–714 (Elsevier, 2014).
Matucci, A., Vultaggio, A., Maggi, E. & Kasujee, I. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Are we asking the right question? Respir. Res. 19, 113 (2018).
Sacco, O. et al. Total and Allergen-specific IgE levels in serum reflect blood eosinophilia and fractional exhaled nitric oxide concentrations but not pulmonary functions in allergic asthmatic children sensitized to house dust mites. Pediatr. Allergy Immunol. 14, 475–481 (2003).
Boudier, A. et al. Data-driven adult asthma phenotypes based on clinical characteristics are associated with asthma outcomes twenty years later. Allergy 74, 953–963 (2019).
Acknowledgements
We thank both Dr. Chin-Hao Chang, Ying-Ting Chao, Tse-Le Huang, and Yu-Han Chang from the Department of Medical Research, National Taiwan University Hospital, for invaluable assistance with statistical consultation, analysis, and model development/validation.
Author information
Authors and Affiliations
Contributions
J.-H.L. conceptualized and designed the study, drafted the initial manuscript, and critically reviewed and carefully revised the manuscript. A.-L.C., S.-Y.H., and K.-Y.C. collected data, carried out the initial interpretation, and reviewed and revised the manuscript. B.-L.C., Y.-H.Y., Y.-T.L., L.-C.W., H.-H.Y., and Y.-C.H. supervised data interpretation, and reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
This study was conducted in compliance with the protocol for good clinical practices and the principles of the Declaration of Helsinki. The informed consents were obtained from all participants and/or their legal guardians.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lee, JH., Lin, YT., Chu, AL. et al. Predictive characteristics to discriminate the longitudinal outcomes of childhood asthma: a retrospective program-based study. Pediatr Res 92, 1357–1363 (2022). https://doi.org/10.1038/s41390-022-01956-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-01956-6