Abstract
Objective
Furosemide renal clearance is slow after very preterm (VP) birth and increases with postnatal maturation. We compared furosemide dose frequency and total daily dose between postmenstrual age (PMA) groups in VP infants.
Study design
Observational cohort study of VP infants exposed to a repeated-dose course of furosemide in Pediatrix neonatal intensive care units (NICU) from 1997 to 2016.
Results
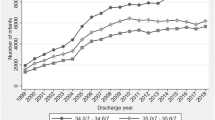
We identified 6565 furosemide courses among 4638 infants. There were no statistically significant differences between PMA groups on the odds of receiving more frequent furosemide dosing. Furosemide courses initiated at <28 weeks PMA were associated with a higher total daily dose than those initiated at a later PMA.
Conclusions
Furosemide dosing practices in the NICU are similar across PMA groups, despite maturational changes in drug disposition. Research is needed to identify and test rational dosing strategies across the PMA spectrum for this commonly used but unproven pharmacotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117:1979–87.
Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK, Smith PB, et al. Medication use in the neonatal intensive care unit. Am J Perinatol. 2014;31:811–21.
Laughon MM, Chantala K, Aliaga S, Herring AH, Hornik CP, Hughes R, et al. Diuretic exposure in premature infants from 1997 to 2011. Am J Perinatol. 2015;32:49–56.
Bamat NA, Kirpalani H, Feudtner C, Jensen EA, Laughon MM, Zhang H, et al. Medication use in infants with severe bronchopulmonary dysplasia admitted to United States children’s hospitals. J Perinatol. 2019;39:1291–9.
Mirochnick MH, Miceli JJ, Kramer PA, Chapron DJ, Raye JR. Furosemide pharmacokinetics in very low birth weight infants. J Pediatr. 1988;112:653–7.
Vert P, Broquaire M, Legagneur M, Morselli PL. Pharmacokinetics of furosemide in neonates. Eur J Clin Pharm. 1982;22:39–45.
Peterson RG, Simmons MA, Rumack BH, Levine RL, Brooks JG. Pharmacology of furosemide in the premature newborn infant. J Pediatr. 1980;97:139–43.
Furosemide Injection, USP [package insert]. American Regent Inc: Shirley, NY. 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018579s029lbl.pdf.
Brater DC. Update in diuretic therapy: clinical pharmacology. Semin Nephrol. 2011;31:483–94.
Thompson EJ, Benjamin DK, Greenberg RG, Kumar KR, Zimmerman KO, Laughon M, et al. Pharmacoepidemiology of furosemide in the neonatal intensive care unit. Neonatology. 2020;117:780–4.
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:1623–7.
Spitzer AR, Ellsbury DL, Handler D, Clark RH. The pediatrix babysteps® data warehouse and the pediatrix qualitysteps improvement project system – tools for “meaningful use” in continuous quality improvement. Clin Perinatol. 2010;37:49–70.
BabysSteps and the Clinical Data Warehouse, Pediatrix Medical Group. 2015. https://docplayer.net/5901868-Pediatrix-medical-group-babysteps-and-the-clinical-data-warehouse.html.
Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants: an evidence-based approach. Am J Respir Crit Care Med. 2019;200:751–9.
Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9.
Lasix (furosemide) Tablets 20, 40, and 80 mg [package insert]. Sanofi-Aventis LLC: Bridgewater, NJ. 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/016273s061lbl.pdf.
van den Anker JN. Pharmacokinetics and renal function in preterm infants. Acta Paediatr. 1996;85:1393–9.
Thayyil S, Sheik S, Kempley ST, Sinha A. A gestation- and postnatal age-based reference chart for assessing renal function in extremely premature infants. J Perinatol. 2008;28:226–9.
Miall LS, Henderson MJ, Turner AJ, Brownlee KG, Brocklebank JT, Newell SJ, et al. Plasma creatinine rises dramatically in the first 48 h of life in preterm infants. Pediatrics. 1999;104:1–4.
Mirochnick MH, Miceli JJ, Kramer PA, Chapron DJ, Raye JR. Renal response to furosemide in very low birth weight infants during chronic administration. Dev Pharm Ther. 1990;15:1–7.
Bamat NA, Nelin TD, Eichenwald EC, Kirpalani H, Laughon MM, Jackson WM, et al. Loop diuretics in severe bronchopulmonary dysplasia: cumulative use and associations with mortality and age at discharge. J Pediatr. 2021;231:43–9.
Tuck S, Morselli P, Broquaire M, Vert P. Plasma and urinary kinetics of furosemide in newborn infants. J Pediatr. 1983;103:481–5.
Okazaki K, Fukuoka N, Kuboi T, Unemoto J, Kondo M, Kusaka T, et al. Furosemide clearance in very preterm neonates early in life: a pilot study using scavenged samples. Pediatr Int. 2021:1–6. https://doi.org/10.1111/ped.14735.
Stewart A, Brion LP. Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database Syst Rev. 2011;9:CD001817.
Greenberg RG, Gayam S, Savage D, Tong A, Gorham D, Sholomon A, et al. Furosemide exposure and prevention of bronchopulmonary dysplasia in premature Infants. J Pediatr. 2019;208:134–40.
Funding
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development grant K23HD10165 to NAB and National Heart, Lung, and Blood Institute Award K24HL143283 to MML. The funding sources had no role in the study design; the collection, analysis, and interpretation of the data; the writing of the report or the decision to submit for publication. NAB wrote the first draft of the manuscript. No compensation honorarium, grant, or other forms of payment was given to produce the manuscript.
Author information
Authors and Affiliations
Contributions
Study conception: NAB, EJT, RGG, SAL, AFZ, ECE, MML. Study design: NAB, EJT, RGG, PBS, MML. Data acquisition: EJT, RGG, VNT, RHC, CPH. Data analysis: NAB, EJT, RGG, MML. Data interpretation: all authors. Drafting the work: NAB, EJT, RGG, MML. Revising it for critically important intellectual content: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bamat, N.A., Thompson, E.J., Greenberg, R.G. et al. Association between postmenstrual age and furosemide dosing practices in very preterm infants. J Perinatol 42, 461–467 (2022). https://doi.org/10.1038/s41372-022-01320-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01320-w