Life is complicated. Even the smallest cells contain a mind-blowing assortment of chemical reactions that allow them to thrive in a chaotic landscape.
If we want to know where to draw the line between life and bubbles of stale old organic soup, it helps to strip away the non-essential extras to expose the core components, and then map how each of them works.
This has been the goal of biochemists for a number of years, who have, over the years, succeeded in designing some surprisingly basic organisms that barely cling to life in a laboratory.
Now, scientists from the J. Craig Venter Institute and the University of Illinois Urbana-Champaign in the US, and the Technische Universität Dresden in Germany, have taken the next step and constructed a detailed simulation of their latest minimalist microbe.
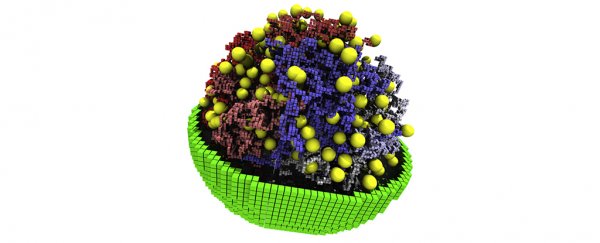
"What's new here is that we developed a three-dimensional, fully dynamic kinetic model of a living minimal cell that mimics what goes on in the actual cell," says University of Illinois Urbana-Champaign chemist Zaida Luthey-Schulten.
Luthey-Schulten led a team of researchers in analyzing the diverse genetic, metabolic, and structural changes that take place in a replicating culture of synthetic bacteria called JCVI-syn3A.
Simulating the workings of the most basic of organisms, such as species of Mycoplasma or the common microbe Escherichia coli, still requires a few mathematical fudge factors to broadly model the operations of numerous sub-systems. Weaving together the full range of detailed descriptions of everything from the genes up and nutrients down just hasn't been possible, even for these comparatively simple bacteria.
In the early 2000s, researchers at the J. Craig Venter Institute removed as many genes as they could from Mycoplasma mycoides, leaving a version that stood right on the brink of survival.
This synthetic life form, called JCVI-syn1.0, was soon superseded by something even more basic. JCVI-syn3.0.
This updated version contains just 531,000 bases divided among 473 genes. With all of its nutrient needs provided by the laboratory, its bare-bones genome is left to take care of replicating and growing and little else.
Still, JCVI-syn3.0 isn't exactly consistent in its growth, producing a confusing diversity of shapes in its progeny. A few genes were popped back in, resulting in the latest version of the minimal cell: JCVI-syn3A.
Its creators have a solid idea of what genes their synthetic cell contains, though are still working out exactly what each one does.
To make things even more difficult, it's vital knowing how each atom and molecule diffuses through the cell, a description that requires heavy-duty computing power to simulate.
"We developed a three-dimensional, fully dynamic kinetic model of a living minimal cell," says Luthey-Schulten.
"Our model opens a window on the inner workings of the cell, showing us how all of the components interact and change in response to internal and external cues. This model – and other, more sophisticated models to come – will help us better understand the fundamental principles of life."
The simulation confirmed a few suspicions, however, such as the fact most of the minimalist cell's energy went towards dragging in essential materials across the membranes.
It also gave an accurate description of the timelines of genetic and metabolic reactions, explaining relationships between the rate of production of lipids and proteins in the membrane and changes in the cell's shape.
Since JCVI-syn3A are essentially pared-down versions of a naturally occurring organism, they're just one example of how to minimalize the functions of biology. Life is nothing if not creative in how it overcomes obstacles to survival.
Now that we have a proven model for simulating JCVI-syn3A's growth and development, researchers can build up its complexity again to determine how different genes add to its function.
We might expect new 'lite' versions of not just M. mycoides, but other organisms in the near future. If not completely novel synthetic life forms.
Life might still be complicated, but it just got a whole lot easier to study.
This research was published in Cell.