Abstract
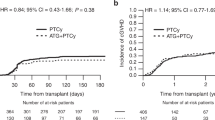
The disease-specific impact of anti-thymocyte globulin (ATG) in allogeneic hematopoietic cell transplantation (allo-HCT) has not been determined. We retrospectively assessed the impact of ATG in allo-HCT using nationwide registry data from the Japan Society for Transplantation and Cellular Therapy. We included patients who received their first allo-HCT between 2007 and 2018 for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), or malignant lymphoma (ML). In total, 8747 patients were included: 7635 patients did not receive ATG and 1112 patients received ATG as GVHD prophylaxis. The median follow-up period of surviving patients was 1457 days. There was no significant impact of pretransplant ATG on the OS or NRM rates in patients with ALL, AML, or ML. In patients with MDS, the probability of 3-year OS was 53.3% in the non-ATG group and 64.2% in the ATG group (P = 0.001). The cumulative incidence rates of relapse and NRM at 3 years were 14.2% and 30.3% (95% CI 27.2–33.3%), respectively, in the non-ATG group and 17.1% and 18.1% in the ATG group (P = 0.15 and P < 0.001). The same finding was observed in a propensity-score matched cohort. Our study suggests that the clinical benefit of ATG could vary among hematological diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–67.
Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. Prospective, Randomized, Double-Blind, Phase III Clinical Trial of Anti-T-Lymphocyte Globulin to Assess Impact on Chronic Graft-Versus-Host Disease-Free Survival in Patients Undergoing HLA-Matched Unrelated Myeloablative Hematopoietic Cell Transplantation. J Clin Oncol. 2017;35:4003–11.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64.
Finke J, Schmoor C, Bethge WA, Ottinger H, Stelljes M, Volin L, et al. Long-term outcomes after standard graft-versus-host disease prophylaxis with or without anti-human-T-lymphocyte immunoglobulin in haemopoietic cell transplantation from matched unrelated donors: final results of a randomised controlled trial. Lancet Haematol. 2017;4:e293–e301.
Kroger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N. Engl J Med. 2016;374:43–53.
Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol. 2016;17:164–73.
Gagelmann N, Ayuk F, Wolschke C, Kroger N. Comparison of different rabbit anti-thymocyte globulin formulations in allogeneic stem cell transplantation: systematic literature review and network meta-analysis. Biol Blood Marrow Transpl. 2017;23:2184–91.
Oostenbrink LVE, Jol-van der Zijde CM, Kielsen K, Jansen-Hoogendijk AM, Ifversen M, Müller KG, et al. Differential Elimination of Anti-Thymocyte Globulin of Fresenius and Genzyme Impacts T-Cell Reconstitution After Hematopoietic Stem Cell Transplantation. Front Immunol. 2019;10:315.
Woo GU, Hong J, Kim H, Byun JM, Koh Y, Shin DY, et al. Preconditioning Absolute Lymphocyte Count and Transplantation Outcomes in Matched Related Donor Allogeneic Hematopoietic Stem Cell Transplantation Recipients with Reduced-Intensity Conditioning and Antithymocyte Globulin Treatment. Biol Blood Marrow Transpl. 2020;26:1855–60.
Heelan F, Mallick R, Bryant A, Radhwi O, Atkins H, Huebsch L, et al. Does Lymphocyte Count Impact Dosing of Anti-Thymocyte Globulin in Unrelated Donor Stem Cell Transplantation? Biol Blood Marrow Transpl. 2020;26:1298–302.
Admiraal R, Nierkens S, de Witte MA, Petersen EJ, Fleurke GJ, Verrest L, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: a multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. 2017;4:e183–e191.
Admiraal R, van Kesteren C, Jol-van der Zijde CM, Lankester AC, Bierings MB, Egberts TC, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. 2015;2:e194–203.
Atsuta Y, Suzuki R, Yoshimi A, Gondo H, Tanaka J, Hiraoka A, et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol. 2007;86:269–74.
Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transpl. 2009;15:367–9.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15:825–8.
Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991;28:250–9.
Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706.
Fine JP, Gray RJ. Fine JP, Gray RJA proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. 94
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Yuan J, Pei R, Su W, Cao J, Lu Y. Meta-analysis of the actions of antithymocyte globulin in patients undergoing allogeneic hematopoietic cell transplantation. Oncotarget. 2017;8:10871–82.
Nagler A, Dholaria B, Labopin M, Socie G, Huynh A, Itälä-Remes M, et al. The impact of anti-thymocyte globulin on the outcomes of Patients with AML with or without measurable residual disease at the time of allogeneic hematopoietic cell transplantation. Leukemia. 2020;34:1144–53.
Rubio MT, D’Aveni-Piney M, Labopin M, Hamladji RM, Sanz MA, Blaise D, et al. Impact of in vivo T cell depletion in HLA-identical allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission conditioned with a fludarabine iv-busulfan myeloablative regimen: a report from the EBMT Acute Leukemia Working Party. J Hematol Oncol. 2017;10:31.
Wang H, Liu H, Zhou JY, Zhang TT, Jin S, Zhang X, et al. Antithymocyte globulin improves the survival of patients with myelodysplastic syndrome undergoing HLA-matched unrelated donor and haplo-identical donor transplants. Sci Rep. 2017;7:43488.
Wang C, Yang Y, Gao S, Chen J, Yu J, Zhang H, et al. Immune dysregulation in myelodysplastic syndrome: clinical features, pathogenesis and therapeutic strategies. Crit Rev Oncol Hematol. 2018;122:123–32.
Nakane T, Fukuda T, Kanda J, Taniguchi S, Eto T, Ohashi K, et al. Age influences post-GVHD non-relapse mortality in adults with acute GVHD of varying severity following allogeneic hematopoietic cell transplantation. Leuk Lymphoma. 2015;56:1–29.
MacMillan ML, DeFor TE, Weisdorf DJ. What predicts high risk acute graft-versus-host disease (GVHD) at onset?: identification of those at highest risk by a novel acute GVHD risk score. Br J Haematol. 2012;157:732–41.
Murata M, Nakasone H, Kanda J, Nakane T, Furukawa T, Fukuda T, et al. Clinical factors predicting the response of acute graft-versus-host disease to corticosteroid therapy: an analysis from the GVHD Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transpl. 2013;19:1183–9.
Shichijo T, Fuji S, Nagler A, Bazarbachi A, Mohty M, Savani BN. Personalizing rabbit anti-thymocyte globulin therapy for prevention of graft-versus-host disease after allogeneic hematopoietic cell transplantation: is there an optimal dose? Bone Marrow Transpl. 2020;55:505–22.
Acknowledgements
This study was partly supported by Health, Labor and Welfare Sciences Research Grants (20FF1002).
Author information
Authors and Affiliations
Contributions
SF, TH, TK, and HN designed the research, organized the project, and wrote the paper. SF performed the statistical analyses. ND, MS, YK, NU, TA, MT, TE, KM, TK, YO, YK, OM, TF, and YA gathered the data. All authors contributed to the final version of the manuscript and have approved it for submission for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fuji, S., Hirakawa, T., Takano, K. et al. Disease-specific impact of anti-thymocyte globulin in allogeneic hematopoietic cell transplantation: a nationwide retrospective study on behalf of the JSTCT, transplant complications working group. Bone Marrow Transplant 57, 479–486 (2022). https://doi.org/10.1038/s41409-022-01569-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-022-01569-x
This article is cited by
-
Impact of anti-thymocyte globulin on survival outcomes in female-to-male allogeneic hematopoietic stem cell transplantation
Scientific Reports (2023)
-
Allogeneic transplantation of bone marrow versus peripheral blood stem cells from HLA-identical relatives in patients with myelodysplastic syndromes and oligoblastic acute myeloid leukemia: a propensity score analysis of a nationwide database
Annals of Hematology (2023)