Abstract
For the first time, lasing at NV− centers in an optically pumped diamond sample is achieved. A nanosecond train of 150-ps 532-nm laser pulses was used to pump the sample. The lasing pulses have central wavelength at 720 nm with a spectrum width of 20 nm, 1-ns duration and total energy around 10 nJ. In a pump-probe scheme, we investigate lasing conditions and gain saturation due to NV− ionization and NV0 concentration growth under high-power laser pulse pumping of diamond crystal.
Similar content being viewed by others
Introduction
Color centers in diamond exhibit outstanding spectroscopic properties1 with photoluminescence spectra covering visible and near-IR wavelength ranges2,3,4,5,6. Optical transparency of diamond and its excellent mechanical, thermal, and optical characteristics make it a promising host material for color-center photon source7,8. One of the most studied color centers in diamond, the negatively charged nitrogen-vacancy (NV−) center, is a unique solid-state quantum system exhibiting high quantum efficiency at room temperature. It can be coupled to dielectric cavities and plasmonic resonators in order to create single-photon emitters applicable in quantum photonic technologies9,10,11. Laser initialization, changing and reading the spin state of an NV− electron allows to control the state of a quantum system and turn NV− centers in diamond into a promising platform for optical quantum computing12,13,14,15,16,17,18,19, quantum metrology, sensing and visualization, fluorescent magnetometry and laser threshold magnetometry that requires a CW laser constructed from the NV− centers20,21,22,23,24,25,26.
For decades, diamond crystals with nitrogen-vacancy centers have been considered as a potential lasing medium2,27,28. Nitrogen vacancy color centers exist in two states: negatively charged—NV− (with its zero-phonon line at 637 nm) and neutral—NV0 (with its zero-phonon line at 575 nm)1. Both states support strong electron–phonon interactions that result in a very broad phonon sideband (PSB) emission1,11. Exhibiting large emission and absorption cross sections, high quantum efficiency and a broad gain band, diamond with NV centers can be considered as a promising solid state active media for tunable and femtosecond lasers in the red and near infrared spectral regions29.
Monocrystalline samples of IIa-type diamond with NV color centers created by means of high pressure and high temperature synthesis (HPHT) are currently available on the market29,30,31. Spectroscopic characteristics of NV− centers in diamond were investigated in27; but, stimulated emission or lasing at NV centers in diamond have not been achieved in that work. Stimulated emission at wavelengths in the PSB of NV− zero-phonon line (ZPL) was registered in28 through lock-in detection consistent with a simultaneous reduction in spontaneous emission under a CW optical pump at 532 nm. In the recent work32 the amplification of 721-nm laser radiation by stimulated emission from NV− centers in diamond inside an optical fiber cavity was demonstrated. However, a lasing at NV− centers in diamond have not been realized yet.
In this work, we investigated in detail spectral properties of the HPHT diamond sample with two growth zones exhibiting different NV0 to NV− concentration ratios. Sample photoluminescence was enhanced by CW and pulsed lasers at various wavelengths. A direct pump-probe method was used for registration of stimulated emission from the diamond sample. We demonstrate that the diamond sample zone with a predominance of NV0 centers exhibits only pump-induced absorption of the probe, while the growth zone with a high concentration of NV− centers provides the probe amplification. However, we detect a saturation of the probe amplification and probe absorption that appears due to NV− ionization and NV0 formation at sufficiently high pump rates. The maximal detected gain coefficient is about 1.5 cm–1 that allows us to reach lasing conditions in the diamond sample zone with a predominant NV− concentration and, for the first time, we achieve a laser generation at NV− color centers in diamond.
Results
Spectroscopic characterization of the diamond sample
An investigated diamond sample is a 4 × 3 × 0.25 mm plate containing two growth zones (Fig. 1) with significantly different NV0 to NV− concentration ratios. The largest part of the sample (zone S1) mostly contains neutral NV0 centers. In a narrow (about 250 μm) dark-colored region at the edge of the sample (zone S2) the concentration of NV− centers turned out to be significantly higher than the NV0 concentration. Two lateral faces of the sample were polished at the Brewster’s angle (See Methods for more details).
The absorption spectra after Fresnel correction are given in Fig. 2a in the units of cm−1 (gray curve for S1 and black curve for S2 sample zones, respectively). The absorption coefficient \(\alpha (\lambda )=\frac{1}{d}{{{{{\rm{ln}}}}}}\frac{{I}_{0}(\lambda )}{I(\lambda )}\) was obtained by illuminating the sample by a tungsten incandescent lamp with incident intensity I0(λ) and transmitted intensity I(λ) through the sample with thickness d = 0.25 mm for each zone. Transmitted intensity I(λ) was measured at room temperature employing an Ocean Optics FLAME-S-XR1 spectrometer with optical resolution 1.69 nm FWHM and 200-μm fiber input for light collection. The obtained optical absorption spectra are typical for type IIa diamonds1,6.
a Absorption spectrum of S1 (gray curve) and S2 (black curve) zones of diamond sample. The spectra were recorded at room temperature. Normalized photoluminescence spectra of S1 (b) and S2 (c) zones under continuous excitation at various wavelengths: 532 nm (green curve), 450 nm (blue curve), and 400 nm (violet curve). For each pump wavelength the spectra collected from both zones were normalized on the S1-zone spectral amplitude at 575 nm that corresponds to the NV0 zero-phonon line. The photoluminescence spectra were collected from the sample excitation surface, i.e. the pump lasers and the recording spectrometer were located on the same side of the sample.
The S1-zone absorption spectrum has a maximum at 575 nm and a width of about 150 nm (Fig. 2a, gray curve), which corresponds to the characteristic absorption spectrum of NV0 centers in diamond27,28. The S2-zone absorption spectrum contains a pronounced absorption band from 500 to 660 nm with a maximum around 569 nm due to the domination of NV– centers in this zone (Fig. 2a, black curve). At the peak NV– absorption wavelength the absorption coefficient reaches 14 cm–1, which is an order of magnitude less than the NV–-containing diamond sample absorption coefficient measured in27. We observed strong blue absorption in the spectral range from 400 to 495 nm (Fig. 2a, black curve) that indicated a significant amount of nitrogen atoms in the S2 zone33. The nitrogen concentration 3.1 × 1019 cm−3 (175 ppm) in S2 zone was measured employing Bruker Vertex 70 FTIR spectrometer combined with a micro-scope Hyperion 2000. Electrons of nitrogen donor atoms (C-centers) are captured by NV0 centers, thereby changing their state to NV–34. Thus, the presence of nitrogen atoms in the S2 zone naturally leads to an increase in the NV– concentration and, we believe, is the key difference between the zones S1 and S2.
We used three CW lasers with a power of about 10 mW at wavelengths of 400, 450, and 532 nm to illuminate the sample. The sample was mounted on a vertical translation stage with a micrometric step resolution allowing to operate with sample zones S1 and S2 without any readjustment of the experimental set-up. The photoluminescence spectra were collected from the illuminated sample surface, i.e. the incident laser beam and the recording spectrometer were located on the same side of the sample. Normalized photoluminescence spectra of S1 and S2 zones recorded at room temperature employing the Ocean Optics FLAME-S-XR1 spectrometer are given in Fig. 2b and c, respectively. For each excitation wavelength, the spectra collected from both zones were normalized on the S1-zone spectral amplitude at 575 nm that corresponds to the NV0 ZPL in diamond. The photoluminescence spectra of the zone S1 exhibit a strong NV0 centers ZPL at 575 nm and its PSB emission with a maximum at 609 nm at all considered excitation wavelengths (Fig. 2b). ZPL of NV– centers at 638 nm and its PSB emission with a maximum at 680 nm are presented in the photoluminescence spectra of both zones under 532-nm excitation (Fig. 2b, c green curves). The photoluminescence spectra under 400- and 450-nm excitation for zone S1 are identical (Fig. 2b blue and violet curves) and have much higher amplitudes than the photoluminescence spectra from S2 zone (Fig. 2c blue and purple curves) because both excitation wavelengths fall in the NV0 absorption region and are not absorbed by the NV– centers.
The decay time of the spontaneous emission signal integrated over in the wavelength range 670–680 nm was found to be τ21 = 9.3 ± 0.3 ns for the S2 zone with a predominant concentration of NV− color centers. We employed a fast optoelectronic converter to measure τ21 (See Methods for more details). The obtained decay time is close to the NV− excited state lifetime given in a number of other works: 8 ± 1 ns in35, 7.8 or 12 ns depending on the spin state of the diamond defect36, 12 and 13 ns in27,28 and29, respectively.
Based on photoluminescence spectra of the sample zone S2 we calculate emission cross section σem at the center of the emission band λem = 680 nm (See Methods for details). The estimated value reaches σem = 4.9·10−17 cm2 that is close to the emission cross-section values 4.3·10−17 cm2 and 3.6·10−17 cm2 reported in27 and35, respectively, and it is one order below the value 3.2·10−16 cm2 given in29. Corresponding absorption cross section at the center of the absorption band λabs = 569 nm of zone S2 (See Methods for details) is σabs = 5.7·10−17 cm2 that matches well with the absorption cross-section values reported in following works: 2.8·10−17 cm2 27, (3.1 ± 0.8)·10−17 cm2 37, and (9.5 ± 2.5)·10−17 cm2 38. Using the obtained absorption cross-section σabs and corresponding absorption coefficient α from Fig. 2a, the concentration \({N}_{N{V}^{-}}=\frac{\alpha }{{\sigma }_{abs}d}\) of NV− centers is found to be ~2.5·1017 cm−3 (1.4 ppm) in the S2 zone. This value lies in the range of the NV– concentrations corresponding to the lasing conditions predicted in28. Note that in work27, where lasing was not achieved, the concentration of NV– color centers was 4.5·1018 cm−3, which is an order of magnitude higher than the concentration of NV– centers in the S2 zone of our sample.
Pump-saturation effect of photoluminescence
To investigate a photoluminescence at high intensity pump rate, we used a picosecond Nd:YAG laser designed in our laboratory that delivers a train of 150-ps pulses delayed by 4.4 ns from each other with possibility of a high contrast selection of a single pulse39. Total energy of the 532-nm pulses train has reached 400 µJ and could be attenuated by set of filters introduced in the optical path. The average light field intensity of pulses train focused into a spot with a diameter of 120 μm (FWHM) reaches about 5·107 W/cm2, and the peak intensity of single pulse is 3·109W/cm2.
We have detected experimentally that for both S1 and S2 sample zones the spectral amplitude of NV0 ZPL at 575 nm grows under pump energy increase (Fig. 3a and b, respectively). In the zone S1 the shape of the luminescence spectrum has characteristic features defined by NV0 PSB for all considered incident pulse train energies, while NV– ZPL at 638 nm and its PSB are weakly pronounced. In the zone S2 NV– PSB defines the luminescence spectrum shape, which has a maximum at ~680 nm for low pump energies (Fig. 3b, black curve). The pump energy increase from 0.1 µJ up to 400 µJ leads to the enhancement of the NV– centers photo-ionization process and corresponding NV0 concentration growth in the S2 zone. As a result, we observe a saturation of the NV– contribution and appearance of the NV0 ZPL and PSB in the range of 575–630 nm in the photoluminescence spectrum (Fig. 3b, blue and red curves). The observed pump-saturation effect of NV– photoluminescence is in a good agreement with the works27,40,41,42, where the role of the NV– photo-ionization process under pump power increase was investigated.
Photoluminescence spectra of (a) S1 and (b) S2 sample zones under excitation of 532-nm pulsed pump with the pulse train total energy of 1.3 µJ (black curve), 15 µJ (blue curve), 400 µJ (red curve). The dip in the spectra around the pump wavelength of 532 nm is related to the Thorlabs NF533-17 Notch filter present in the scheme. The spectra were recorded in transmission configuration; the pump laser and the recording spectrometer were located on opposite sides of the sample.
Stimulated emission from NV– centers in diamond
We employed a direct pump-probe method for stimulated emission registration from the S1 and S2 zones of the diamond sample (Fig. 4). A 675-nm Thorlabs HL6750MG CW laser diode with 100-mW power was used to probe the sample. In the first series of experiments, the sample was placed perpendicularly to the probe beam at a 150-mm distance from the laser diode. An 11-mm lens creates a 50-µm (FWHM) probe beam waist at the sample surface (Fig. 4, inset 1). A vertical translator with a micrometer resolution provided an opportunity to switch between the studied sample zones S1 and S2. In this case, to pump the sample in a single-pulse regime, we cut the central laser pulse from a pulse train of 150-ps pulses39. The energy of the 532-nm single pulse was controlled by attenuating filters in the range from 0.6 to 15 µJ. The waist diameter of the pump beam focused by a 150-mm lens on the sample surface was about 120 μm (FWHM). An interference filter for 675–680 nm was placed at the entrance of a collimator Thorlabs F220FC-1064, which collected the laser diode radiation passed through the sample into the measuring optical fiber. To minimize the influence of spontaneous luminescence, the collecting collimator was located at a distance of about 200 mm from the sample. The spontaneous luminescence level, which enters the collimator without switching on the probe diode, was no more than 5% of the probe signal level and was subtracted when processing the stimulated emission gain. The stimulated emission was detected by a LeCroy WaveMaster 808Zi-A oscilloscope with a LeCroy OE555 optoelectronic converter with 4.5 GHz sample rate, which allows to study in real time the dynamics probe amplification in the sample under picosecond laser pump.
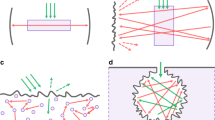
Scheme of a pump-probe experiment for registration of stimulated emission from the diamond sample. We employed transverse probe configuration in which the sample was placed perpendicularly to the probe beam (inset 1) and longitudinal probe configuration in which probe beam was going along the diamond parallel to sample side surfaces (inset 2).
In the second series of experiments a cylindrical lens was employed to create an elliptical pump beam profile illuminating the whole zone S2 with surface area of about 4 × 0.25 mm. Since the pump beam area was increased significantly, we apply the full train of 15 picosecond pulses, instead of a single pump pulse. The sample was placed at the Brewster’s angle to the probe beam, so that the probe beam was going along the diamond parallel to sample side surfaces (Fig. 4, inset 2). Note, that conventional Q-switched nanosecond Nd:YAG lasers usually have a stochastic structure of resonator light field time profile that leads to significant pulse-to-pulse intensity variations with cavity round-trip period features. Such pump pulses cannot allow a controlled uniform pumping of the sample. In opposite, the train of active mode-locked picosecond laser pulses employed in our experiments has high temporal regularity and shot-to-shot repeatability39.
The kinetics of the probe laser diode signal transmitted through the sample zones S1 and S2 are shown in Fig. 5a and b, respectively. The signal was normalized to the level of the unperturbed laser diode signal transmitted through the sample in the absence of a pump pulse. The 532-nm picosecond pump pulse arrives at moment in time t = 0, and leads to a disturbance of the detected 675-nm signal.
Kinetics of the laser diode signal in a wavelength range from 675 nm to 680 nm transmitted through the sample zones S1 (a) and S2 (b) pumped by a single 150-ps 532-nm pulse at various energies: 0.6 µJ (black curve), 2.3 µJ (blue curve), 6.6 µJ (green curve), 15 µJ (red curve) in case of transverse probe configuration (Fig. 4, inset 1). с Kinetics of the 675-nm laser diode signal transmitted through the sample zone S2 pumped by a train of 150-ps 532-nm laser pulses at various energies: 61 µJ (blue curve), 176 µJ (green curve), and 400 µJ (red curve) in case of longitudinal probe configuration (Fig. 4, inset 2). Corresponding train of pump pulses with normalized amplitudes is plotted as a black curve. The front weak pulses of the train were dumped by a pulse selector.
In the S1 zone, characterized by the presence of the NV0 centers, the appearance of a pump pulse leads to an attenuation of the probe signal at all the considered pump energies (Fig. 5a). The leading edge of the transient response has a duration of less than 100 ps, which corresponds to the profile of the pump pulse. The relaxation process of the pump-induced absorption is essentially nonlinear and its characteristic time depends on the energy of the pump pulse. For pump energy below 1 µJ, the relaxation time is about 20 ns; for higher pump energies, the complete relaxation was not reached at times up to 10 μs. In this case, the relaxation process cannot be decomposed into fast and slow components and looks rather like a multi-particle process with a smooth change in the relaxation rate.
In the S2 zone, characterized by the predominance of the NV– centers, we registered the pump induced amplification of the probe signal (Fig. 5b). For pump energies below 7 µJ, the relaxation process of the pump-induced amplification has a characteristic time about 10 ns (Fig. 5b, black, blue and green curves), which is close to the lifetime of the NV– excited state, since the probe beam has a relatively weak intensity. The maximum registered gain is about 1.05 at a pump pulse energy of 6.6 µJ. Taking the sample depth as 0.25 mm and stimulated emission cross-section σem = 4.9·10−17 cm2 (see the first section), the concentration of the pumped NV– centers reaches 0.4·1017 cm−3 that is about 16% from the total concentration of the NV– centers in the S2 zone.
At a pump energy of 15 µJ, the ionization process of the NV– centers and corresponding growth of the NV0 concentration leads to a transition from the probe signal amplification to the probe signal absorption that occurs on a 1.5-ns time scale (Fig. 5b, red curve). Signal absorption at this pump energy indicates the formation of free carriers upon ionization of NV- and NV0 centers in the S2 zone (see Fig. 3b). The dynamics of the induced-absorption relaxation process is identical to the dynamics in the S1 zone. Thus, the process of induced absorption correlates with the presence of NV0 centers in the studied sample, regardless of whether they were present initially or were formed as a result of pulse-induced ionization of NV– centers.
From the obtained kinetics of the probe signal, it is clear that there is a pump rate threshold, an exceeding of which leads to saturation of probe gain and further switch to an attenuation of the probe signal. Due to the demonstrated pump-saturation behavior of the probe gain, we consider a side pump configuration along the sample allowing us to reach a higher gain level. A cylindrical lens was employed to create an elliptical pump beam profile illuminating the crystal zone S2 with surface area of about 4 × 0.25 mm. Since the pump beam area was increased significantly, we apply the full train of 15 picosecond pulses, instead of a single pump pulse. The sample was placed at the Brewster’s angle to the probe beam, so that the probe beam was going along the diamond parallel to sample side surfaces (Fig. 4, inset 2). The S1 zone of the sample was not considered in this series of experiments, due to no stimulated emission was observed in this zone.
Recorded kinetics of the probe signal transmitted through the sample shown in Fig. 5c for various energies of a multi-pulse pump. At low pump energy of 61 µJ, the probe amplification profile repeats the intensity profile of the pump pulse train (Fig. 5c, blue curve). At higher pump energy of 176 µJ, weak probe absorption appears at the end of the pump train (Fig. 5c, green curve). A saturation of probe amplification followed by NV–-ionization induced absorption occurs at sufficiently high pump energy of 400 µJ (Fig. 5с red curve). Further increase of the pump energy will lead to an over-threshold pumping regime that does not provide optimal lasing conditions. The maximum achieved gain is 1.8 (gain coefficient 1.5 cm–1), which is quite sufficient for stable lasing conditions. The corresponding concentration of exited NV– centers reaches 0.3·1017 cm–3, that is about 12% from the total concentration of the NV– centers in the S2 zone.
Lasing on NV− centers in diamond
Herein we report the scheme of the resonator, used for demonstration of the lasing on NV– centers in diamond, and the characterization of the lasing pulses. The resonator consists of two identical flat mirrors with reflection coefficient 95% in the range of wavelengths from 700 to 750 nm. The diamond sample was aligned at the Brewster’s angle in resonator that provides a lasing direction along the sample. In order to compensate the beam divergence and to increase the resonator stability, a BK7 spherical lens with focal length of 15 mm was introduced in the resonator at the Brewster’s angle to the beam axis (Fig. 6a). The cavity length was chosen to provide a symmetrical mode on one of the mirrors (Fig. 6d). We were operating with the sample zone S2, in which the stimulated emission has been obtained. A full train of 150-ps 532-nm laser pulses was used to pump the sample. As in the previous section, the cylindrical lens was employed for side illumination of the whole S2 zone of the sample.
A laser generation at NV– color centers in diamond was obtained under pump energies of ps-pulse train above 20 µJ. Dependence of output laser pulses energy (sum of energies from two couplers) on pump energy is presented in Fig. 7a. The maximal energy of the achieved laser pulses reaches 10.4 nJ (5.2 nJ at each output coupler) under 260-µJ pump. With a further increase of pump rate, the output pulses energy decreases due to NV centers ionization and formation of an induced plasma. Spatial profiles of lasing mode were recorded at 200-mm distances from each output coupler by the Ophir SP620U CCD camera and are shown in Fig. 6b and c. The polarization measurements show that the laser beam is linear polarized according to the Brewster’s angle of diamond sample.
Laser pulses energy (sum of energies from two couplers) versus the pump (a), time profiles of lasing pulses (b), and lasing spectra (c) at various pump pulse train energies: 35 µJ (black curve), 73 µJ (violet curve), 140 µJ (blue curve), 260 µJ (green curve) and 400 µJ (red curve). Black dashed curves: train of pump pulses with normalized amplitudes (a) and photoluminescence spectra of S2 zones of diamond sample under continuous excitation at 532 nm (b).
Temporal profiles of the lasing pulse at various pump energies are given in Fig. 7b. The minimal lasing pulse duration of about 1 ns (FWHM) has been achieved for pump energies from 73 µJ to 140 µJ (Fig. 7b violet and blue curves). The active media inversion population changes dramatically on the time scale of a delay between the neighboring pulses in the pump train because the lifetime of the NV– excited state (about 10 ns) is comparable to the 4.4-ns delay between pump pulses in the train. In this case the minimal laser pulse duration can be achieved if the laser generation develops in resonator after the arrival of the second pulse from the pump pulse train. At high pump energies (260 µJ and 400 µJ), the lasing development occurs between the first and the second pump pulses of the train and corresponding lasing pulse time profile consists of several peaks (Fig. 7b red and black curves).
Despite the fact that the fluorescence peak of NV– centers in diamond lies at a wavelength of 680 nm (Fig. 2c), the laser pulse spectrum has a maximum at 720 nm (Fig. 7c). This is not a contradiction, since the gain cross-section spectrum can be obtained by multiplying the fluorescence emission spectrum by a factor of λ5 and, therefore, the gain maximum is shifted to the long-wavelength region43. Similarly, for the well-known Ti:Sapphire laser the fluorescence emission peak is at 750 nm, while the gain maximum is located near 800 nm43.
The laser pulse spectrum width is about 20 nm (FWHM). A lasing process starts from noise in the entire gain band and the pulse rise time in the cavity (~5 ns, 20–30 roundtrips) is sufficiently for lasing spectrum narrowing by about an order in comparison with luminescence spectrum width (Fig. 7c).
Discussion
Comparative spectroscopic studies have proven the possibility of lasing in diamond samples containing NV– color centers with densities about 1017 cm−3. The presence of NV0 centers, regardless of whether they were initially contained in the sample or were formed during the NV– ionization process under a high-intensity laser pumping, leads to a decrease of the stimulated emission and a transition to the probe absorption regime. We conclude that for 532-nm pump wavelength there is an optimal pumping rate at which the highest gain in the NV– diamond is achieved. The maximum reached gain is 1.8 (gain coefficient 1.5 cm–1) when the sample zone containing NV– centers was pumped by a train of picosecond pulses. Stable lasing at a wavelength of 720 nm was achieved in diamond sample under optimal pumping conditions. Compelling experimental evidence on lasing were observed: 10-nJ pulse energy, a near diffraction limited laser beam, linear polarization, nanosecond pulse temporal profile, and lasing spectrum narrowing by about an order in comparison with luminescence spectrum width.
In our vision the next important step in future investigations is CW lasing in diamond samples with NV– color centers. The movement towards the quasi-CW and CW lasing should be carried out by gradually increasing the duration of the pumping pulse, while carefully controlling the gain and induced losses ratio. One of the problems reaching CW lasing is the absorption in the induced plasma during the ionization of the NV centers by pump radiation.
The achieved lasing at NV– color centers in diamond will lead to an enhancement of a broad laser system development based on the NV– diamond active media. A unique combination of properties such as thermal conductivity, low thermal expansion, and mechanical strength makes diamond crystals an excellent candidate for high-power CW and ultrashort lasers.
Methods
Diamond sample preparation
A diamond sample is a 4 × 3 × 0.25 mm plate cut from HPHT diamond subjected to a radiation treatment by electrons accelerated to 3 MeV with a dose of about 1·1018 e−/cm2 and subsequent annealing in vacuum at a temperature of 800 °C for 24 h. Two lateral sides of the sample were polished at 22.5-degrees angle to enable studies of the probe amplification incident on the sample at the Brewster’s angle (67.5 degrees).
The diamond sample has two growth zones with significantly different concentrations of nitrogen, which is incorporated into the diamond lattice during HPHT synthesis. Most of the diamond sample has a nitrogen concentration of less than 1 ppm (zone S1), while the narrow colored area at the edge of the sample has a nitrogen concentration that is about 2 orders of magnitude higher (zone S2). NV complexes are formed in the crystal after irradiation with 3 MeV electrons and annealing in vacuum at 800 °C. In the S1 sample zone with a low nitrogen content, a significant fraction (about a half) of nitrogen atoms have formed impurity-defect complexes (NV centers) with vacancies in a neutral charge state. In the zone of the diamond sample with an increased concentration of nitrogen S2 (colored region), the main part of nitrogen remained in the form of single substitutional atoms, which are a donor impurity. In S2 zone, excess electrons from some of the substitutional nitrogen atoms were captured by NV centers. Thus, in the colored region of sample S2, NV centers in the negative charge state were formed, while keeping a negligible fraction of original NV centers in the neutral charge state.
Emission and absorption cross-sections of the sample
For S2 zone of the sample, we estimate emission and absorption cross-sections based on the spectroscopic sample characteristics (Fig. 2). Here we take the fluorescence quantum efficiency being close to 1. The emission cross section was calculated at the center of the emission band λem = 680 nm assuming a Gaussian emission line shape in the frequency domain44,45,46,47,48:
where emission band is fitted by a Gaussian line shape with a width FWHM Δν = 66 THz that corresponds to Δω = 0.27 eV (2.2·103 cm−1) and ω0 = 1.81 eV (1.46·104 cm–1). The estimated emission cross-section value reaches σem = 4.9·10–17 cm2. We estimate absorption cross section at the center of the absorption band λabs = 569 nm assuming also a Gaussian emission line shape in the frequency domain44,45,46,47,48:
where g1 = 1 and g2 = 2 are degeneracies of ground and excited states, respectively, absorption band is fitted by a Gaussian line shape with a width FWHM Δν = 78 THz\(,\) that corresponds to Δω = 0.33 eV (2.7·103 cm–1) and ω0 = 2.16 eV (1.7·104 cm–1). The estimated absorption cross-section value reaches σabs = 5.7·10–17 cm2.
Data availability
The processed numerical data files are available at the public GitHub repository (https://github.com/VMitrokhin/Diamond-Lasing). All other data that support the plots within this paper and other findings of this study are available from the corresponding author upon reasonable request.
References
Zaitsev, A. M. Optical Properties of Diamond. (Springer-Verlag, Berlin Heidelberg, 2001).
Mironov, V., Martynovich, E. & Grigorov, V. A. Laser materials on the base of a diamond. in Physics and Technology of Diamond Materials 96–111 (Polaron, Moscow, 1994).
Rand, S. C. & DeShazer, L. G. Laser action of H3 color center in diamond (Springer-Verlag, 1985).
Pereira, E. & Santos, L. Dynamics of S3 luminescence in diamond. J. Lumin. 45, 454–457 (1990).
Ditalia Tchernij, S. et al. Fluorine-based color centers in diamond. Sci. Rep. 10, 1–7 (2020).
Walker, J. Optical absorption and luminescence in diamond. Rep. Prog. Phys. 42, 1605–1659 (1979).
Rand, S. C. & DeShazer, L. G. Visible color-center laser in diamond. Opt. Lett. 10, 481 (1985).
Mildren, R. P. & Sabella, A. Highly efficient diamond Raman laser. Opt. Lett. 34, 2811 (2009).
Aharonovich, I., Englund, D. & Toth, M. Solid-state single-photon emitters. Nat. Photonics 10, 631–641 (2016).
Bogdanov, S. I. et al. Ultrafast quantum photonics enabled by coupling plasmonic nanocavities to strongly radiative antennas. Optica 7, 463 (2020).
Pezzagna, S., Rogalla, D., Wildanger, D., Meijer, J. & Zaitsev, A. Creation and nature of optical centres in diamond for single-photon emission-overview and critical remarks. N. J. Phys. 13, 35024 (2011).
OIDA. OIDA Quantum Photonics Roadmap: Every Photon Counts (Optical Society of America, 2020).
Wu, Y., Wang, Y., Qin, X., Rong, X. & Du, J. A programmable two-qubit solid-state quantum processor under ambient conditions. npj Quantum Inf. 5, 1–5 (2019).
Bradley, C. E. et al. A ten-qubit solid-state spin register with quantum memory up to one minute. Phys. Rev. X 9, 031045 (2019).
Pinto, D. et al. Readout and control of an endofullerene electronic spin. Nat. Commun. 11, 1–6 (2020).
McCullian, B. A. et al. Broadband multi-magnon relaxometry using a quantum spin sensor for high frequency ferromagnetic dynamics sensing. Nat. Commun. 11, 1–8 (2020).
Irber, D. M. et al. Robust all-optical single-shot readout of nitrogen-vacancy centers in diamond. Nat. Commun. 12, 532 (2021).
Lühmann, T., John, R., Wunderlich, R., Meijer, J. & Pezzagna, S. Coulomb-driven single defect engineering for scalable qubits and spin sensors in diamond. Nat. Commun. 10, 1–9 (2019).
Orwa, J. O. et al. Fabrication of single optical centres in diamonda review. J. Lumin. 130, 1646–1654 (2010).
Jeske, J., Cole, J. H. & Greentree, A. D. Laser threshold magnetometry. N. J. Phys. 18, 013015 (2016).
Gruber, A. et al. Scanning confocal optical microscopy and magnetic resonance on single defect centers. Science 276, 2012–2014 (1997).
Giovannetti, V., Lloyd, S. & MacCone, L. Advances in quantum metrology. Nat. Photonics 5, 222–229 (2011).
Kuwahata, A. et al. Magnetometer with nitrogen-vacancy center in a bulk diamond for detecting magnetic nanoparticles in biomedical applications. Sci. Rep. 10, 1–9 (2020).
Ariyaratne, A., Bluvstein, D., Myers, B. A. & Jayich, A. C. B. Nanoscale electrical conductivity imaging using a nitrogen-vacancy center in diamond. Nat. Commun. 9, 1–7 (2018).
Liu, G. Q., Feng, X., Wang, N., Li, Q. & Liu, R. B. Coherent quantum control of nitrogen-vacancy center spins near 1000 kelvin. Nat. Commun. 10, 1–8 (2019).
Jensen, K. et al. Cavity-enhanced room-temperature magnetometry using absorption by nitrogen-vacancy centers in diamond. Phys. Rev. Lett. 112, 160802 (2014).
Subedi, S. D. et al. Laser spectroscopic characterization of negatively charged nitrogen-vacancy (NV −) centers in diamond. Opt. Mater. Exp. 9, 2076 (2019).
Jeske, J. et al. Stimulated emission from nitrogen-vacancy centres in diamond. Nat. Commun. 8, 370 (2017).
Vins, V. G. & Pestryakov, E. V. Color centers in diamond crystals: their potential use in tunable and femtosecond lasers. Diam. Relat. Mater. 15, 569–571 (2006).
Rubinas, R. et al. Spin properties of NV centers in high-pressure, high-temperature grown diamond. J. Phys. Commun. 2, 115003 (2018).
Rubinas, O.R. et al. Optimization of the coherence properties of diamond samples with an intermediate concentration of NV centers. Results in Physics 21, 103845 (2021).
Nair, S. R. et al. Amplification by stimulated emission of nitrogen-vacancy centres in a diamond-loaded fibre cavity. Nanophotonics 9, 4505–4518 (2020).
Iakoubovskii, K. & Adriaenssens, G. J. Optical transitions at the substitutional nitrogen centre in diamond. J. Phys. Condens. Matter 12, L77 (2000).
Goss, J. P., Briddon, P. R., Jones, R. & Sque, S. Donor and acceptor states in diamond. Diam. Relat. Mater. 13, 684–690 (2004).
Fraczek, E. et al. Laser spectroscopy of NV− and NV0 colour centres in synthetic diamond. Optical Mater. Express 7, 2571 (2017).
Batalov, A. et al. Temporal coherence of photons emitted by single nitrogen-vacancy defect centers in diamond using optical rabi-oscillations. Phys. Rev. Lett. 100, 077401 (2008).
Wee, T. L. et al. Two-photon excited fluorescence of nitrogen-vacancy centers in proton-irradiated type Ib diamond. J. Phys. Chem. A 111, 9379–9386 (2007).
Chapman, R. & Plakhotnik, T. Quantitative luminescence microscopy on nitrogen-vacancy centres in diamond: saturation effects under pulsed excitation. Chem. Phys. Lett. 507, 190–194 (2011).
Savvin, A., Sirotkin, A., Mitrokhin, V. & Dormidonov, A. Picosecond diode pumped Nd:YAG laser for initiation of localized energetic processes in non-laboratory applications. Proceedings - 2020 7th International Congress on Energy Fluxes and Radiation Effects, EFRE 2020, 1048–1052 (2020).
Aslam, N., Waldherr, G., Neumann, P., Jelezko, F. & Wrachtrup, J. Photo-induced ionization dynamics of the nitrogen vacancy defect in diamond investigated by single-shot charge state detection. N. J. Phys. 15, 013064 (2013).
Hacquebard, L. & Childress, L. Charge-state dynamics during excitation and depletion of the nitrogen-vacancy center in diamond. Phys. Rev. A 97, 063408 (2018).
Subedi, S. D., Fedorov, V. V., Mirov, S. B., Shao, L. & Loncar, M. in Laser Congress 2018 (ASSL) AM6A.11 (Optical Society of America, Boston, Massachusetts, 2018).
Moulton, P. F. Spectroscopic and laser characteristics of Ti:Al2O3. J. Optical Soc. Am. B 3, 125–133 (1986).
Davis, C. C. Lasers and electro-optics, second edition (Cambridge University Press, 2013).
Fowler, W. B. & Dexter, D. L. Relation between absorption and emission probabilities in luminescent centers in ionic solids. Phys. Rev. 128, 2154–2165 (1962).
Koechner, W. Solid-State Laser Engineering (Springer, New York, 2006).
Subedi, S.D. et al. Laser spectroscopy of highly doped NV- centers in diamond. Proc. SPIE 10511, 105112D (2018).
Mooney, J. & Kambhampati, P. Correction to “Get the basics right: Jacobian conversion of wavelength and energy scales for quantitative analysis of emission spectra”. J. Phys. Chem. Lett. 5, 3497 (2014).
Acknowledgements
EL and DG acknowledge the Ministry of Education and Science of Russian Federation for funding the research (the state order, project No 0721-2020-0048).
Author information
Authors and Affiliations
Contributions
A.S., A.D. and E.L. conceived and planned the experiments. A.Y., E.L. and D.G. carried out the spectroscopic characterization of the diamond sample. A.S., A.D., V.M. and S.P. carried out the main experiments. A.Y. and V.V. contributed to diamond sample preparation. A.D., E.L., E.S. and A.S. contributed to the interpretation of the results. A.D. and E.S. took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Nature Communications thanks Deepak Kallepalli, Tomasz Jakubczyk and the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Savvin, A., Dormidonov, A., Smetanina, E. et al. NV– diamond laser. Nat Commun 12, 7118 (2021). https://doi.org/10.1038/s41467-021-27470-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-021-27470-7
This article is cited by
-
Magnetic Resonance Express Analysis and Control of NV− Diamond Wafers for Quantum Technologies
Applied Magnetic Resonance (2024)
-
Cathodoluminescence of Nitrogen-Containing Diamond Samples at Temperatures of 80–800 K
Russian Physics Journal (2023)
-
Superluminescence at Nitrogen-Vacancy Centers in the Synthetic Diamond Pumped by Laser Radiation in the Range of 532−575 nm
Russian Physics Journal (2023)
-
Peculiarities of nitrogen-vacancy centers’ superluminescence in diamond under optical pumping at 532 nm
Applied Physics B (2023)
-
Formation of Germanium–Vacancy Color Centers in CVD Diamond
Journal of Russian Laser Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.