- COVID-19
- Biosimilars
- Cataract Therapeutics
- DME
- Gene Therapy
- Workplace
- Ptosis
- Optic Relief
- Imaging
- Geographic Atrophy
- AMD
- Presbyopia
- Ocular Surface Disease
- Practice Management
- Pediatrics
- Surgery
- Therapeutics
- Optometry
- Retina
- Cataract
- Pharmacy
- IOL
- Dry Eye
- Understanding Antibiotic Resistance
- Refractive
- Cornea
- Glaucoma
- OCT
- Ocular Allergy
- Clinical Diagnosis
- Technology
Durable therapies for diabetic macular edema amid COVID-19 pandemic
Patients’ reluctance to visit a clinic during the COVID-19 pandemic is pushing retina specialists to optimize therapy.
During the earliest stages of the COVID-19 pandemic, some patients with diabetic macular edema (DME) were reluctant to visit the office for therapy or monitoring. The risks of complications secondary to COVID-19 infection are elevated among patients with comorbidities such as diabetes. Further, the high burden of care associated with DME has led to undertreatment among real-world patients1—a dynamic almost certainly exacerbated by the aforementioned reluctance to visit the clinic. Given these facts, retina specialists should strive to optimize therapy that reduces exposure risk and burden while still providing excellent outcomes.
Patients with DME commonly undergo therapy with anti-VEGF agents, focal laser application, or steroids. Retina specialists need to weigh the benefits of each treatment option in light of the ongoing pandemic.
Anti-VEGF therapy
Patients with DME undergoing therapy with anti-VEGF agents may need to make frequent visits to the clinic to get effective outcomes, increasing the risk of exposure and causing inconvenience for those who are of working age. Data show that regardless of the anti-VEGF agent they choose, real-world patients have visual outcomes that are inferior to those of patients in randomized controlled trials.2 In the LUMINOUS trial (NCT01318941), patients who received at least 5 injections per year gained significantly more letters than those who received 4 or fewer injections per year (6.9 vs 0.5).3 These results were underscored by those of a later study by Pitcher et al, who found that patients with DME who received 7 injections gained 8.0 mean letters, whereas those who received less therapy gained only 3.7 letters (P < .001).4
Focal laser application
In a tightly controlled clinical trial, patients return to the clinic for treatment according to the study protocol. However, real-world evidence demonstrates that patients who do not receive frequent treatment struggle to regain vision. Patients and clinicians who wish to avoid frequent office visits might turn to focal laser therapy for DME. Although focal laser is effective in some cases for treating DME, its inability to treat the surrounding tissue creates an environment in which extrafoveal fluid associated with DME resolves while DR remains active in the periphery.
Steroid options
If reducing treatment burden is part of what drives decision-making, then retina specialists may wish to explore steroid therapy. Pivotal trials for both the intravitreal fluocinolone acetonide implant 0.19 mg (Iluvien, Alimera Sciences)5 and the intravitreal dexamethasone implant 0.7 mg (Ozurdex, Allergan)6 showed that sustained-release steroid implants were superior to sham therapy for DME. Ozurdex, which was studied as a 6-month therapy, is approved for the treatment of DME in pseudophakic patients or in those who are scheduled for cataract surgery.7
Iluvien, which is designed to elute fluocinolone acetonide for approximately 36 months, is indicated for patients with DME who have previously been treated with a course of corticosteroids and did not have a clinically significant rise in IOP.8 Often clinicians will administer Iluvien after determining that the patient’s disease responds to corticosteroids, allowing for a longer duration of treatment—and, in the COVID-19 era, reduced risk of transmission in the retina clinic.
Clinicians may wish to consider moving Iluvien further up in their decision tree for the treatment of DME. In a 24-month interim analysis of the prospective, observational, real-world study PALADIN (NCT02424019), investigators found that treatment with Iluvien resulted in a mean visual improvement of 1 to 3 letters, improved central subfield thickness, and fewer DME treatments. Fifty-one percent of patients required no treatment or 1 treatment during the follow-up period and a majority of patients required no rescue treatment at 1 year.9 The case below illustrates how Iluvien therapy may result in no need for further treatment.
Among DME patients who struggle with follow-up appointments, Iluvien therapy may be more effective than anti-VEGF therapy at reducing the risk of vision-threatening complications of proliferative retinopathy. In a time-matched, consecutive series of the aggregated Vestrum database from January 2013 to February 2021, my research team and I found that patients with DME who had at least a 6-month break in follow-up progressed to proliferative diabetic retinopathy at rates of 1% and 10% for those undergoing Iluvien or anti-VEGF therapy, respectively.10 Also, new vitreous hemorrhage was present upon return in more patients undergoing anti-VEGF therapy (6%) compared with Iluvien therapy (< 1%).10
In light of these new data, it is important that clinicians give careful consideration and weigh the high treatment burden of frequent anti-VEGF therapy against the benefits of 36-month sustained treatment with Iluvien. Increasing our field’s use of Iluvien in the treatment of DME may satisfy our patients’ request for fewer office visits while also maximizing patient therapy.
Case
Figure 1. An OCT imaging showed evidence of macula edema. (Images courtesy of Caesar K. Luo, MD, FACS, FASRS)
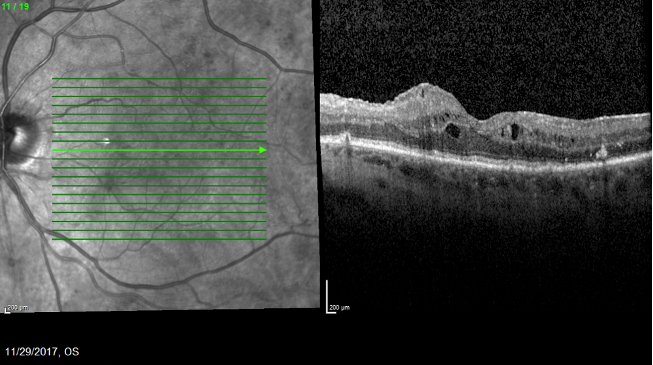
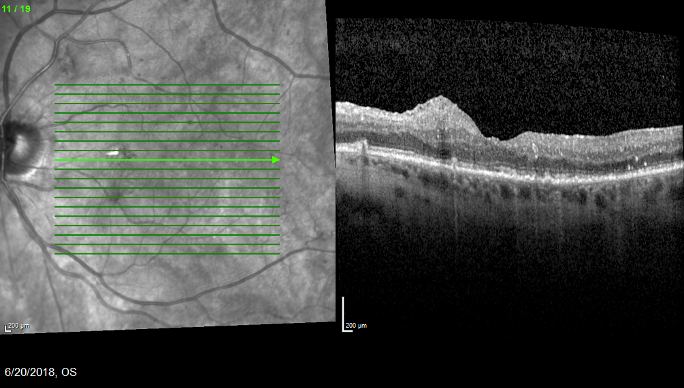
A 73-year-old woman with a history of vitrectomy and membrane peel presented in 2017 with 20/50 VA and DME (Figure 1). The patient was lost to follow-up and returned in 6 months with 20/60 VA and worsened edema. I elected to administer Ozurdex, and she returned in 1 month with 20/40 VA and improved edema (Figure 2).
Figure 2. One month after Ozurdex administration, the patient’s anatomy showed signs of resolution.
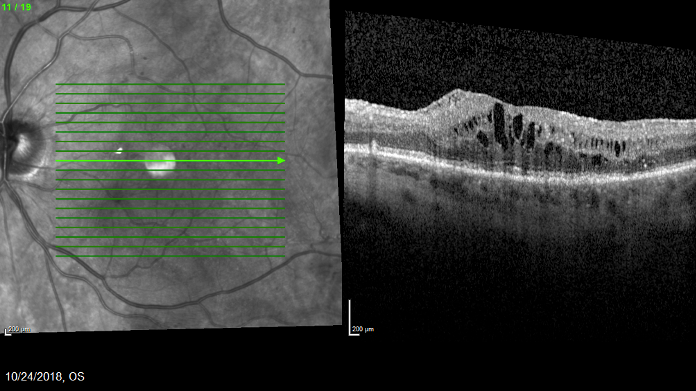
Five months after she received the Ozurdex, her DME had recurred and VA dipped to 20/80 (Figure 3). Given her previous positive functional and anatomic response to steroid therapy, I proceeded with Iluvien administration.
Figure 3. The patient’s edema recurred 5 months after she received Ozurdex. At this appointment, Iluvien was administered.
Her IOP at the time of the Iluvien administration was 18, with a cup to disc ratio of 0.4. She returned in 1 month with 20/40 VA and resolution of the macular edema. At her most recent visit (September 2021), which was 3 years after receiving Iluvien, her VA was 20/25 and her anatomy showed evidence of disease quiescence (Figure 4).
Figure 4. The patient’s anatomy was stable 3 years after Iluvien was administered, and VA was 20/25.
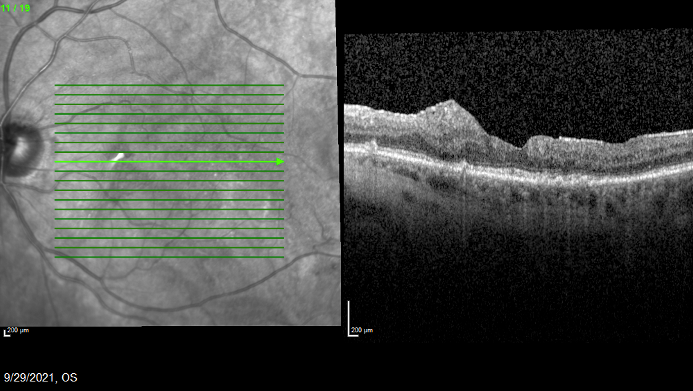
Part of the reason this patient is noteworthy is that she was lost to follow-up for nearly a year during the COVID-19 pandemic, largely due to her discomfort with coming in to the office. Upon her return, her IOP was 15 with a stable cup to disc ratio. During that period, her macular edema was controlled with Iluvien therapy and her VA had actually improved from 20/40 to 20/25.
Caesar K. Luo, MD, FACS, FASRS
e: caesarluomd@gmail.com
Luo serves on the advisory board of Alimera Sciences. He has received research grants from Allergan, and is a speaker for Iridex and Regeneron.
REFERENCES
1. Kiss S, Malangone-Monaco E, Wilson K, et al. Real-world injection frequency and cost of ranibizumab and aflibercept for the treatment of neovascular age-related macular degeneration and diabetic macular edema. J Manag Care Spec Pharm. 2020;26(3):253-266. doi:10.18553/jmcp.2020.19245
2. Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world outcomes of anti-vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmol Retina. 2018;2(12):1179-1187. doi:10.1016/j.oret.2018.06.004
3. Mitchell P, Sheidow TG, Farah ME, et al; LUMINOUS Study Investigators. Effectiveness and safety of ranibizumab 0.5 mg in treatment-naïve patients with diabetic macular edema: results from the real-world global LUMINOUS study. PloS One. 2020;15(6):e0233595. doi:10.1371/journal.pone.0233595
4. Pitcher JD, Moshfeghi AA, Lucas G, Boucher N, Moini H, Saroj N. Evaluation of patients receiving intravitreal antivascular endothelial growth factor for diabetic macular edema in clinical practice in the United States. J Vitreoretin Dis. 2021;5(2):108-113. doi:10.1177/2474126420953067
5. Campochiaro PA, Brown DM, Pearson A, et al; FAME Study Group. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119(10):2125-2132. doi:10.1016/j.ophtha.2012.04.030
6. Boyer DS, Yoon YH, Belfort R Jr, et al; Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904-1914. doi:10.1016/j.ophtha.2014.04.024
7. Ozurdex. Prescribing information. Allergan, Inc; 2014. Accessed December 6, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022315s009lbl.pdf8.
8. Iluvien. Prescribing information. Alimera Sciences, Inc; 2014. Accessed December 6, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/201923s000lbl.pdf
9. Mansour SE, Kiernan DF, Roth DB, et al. Two-year interim safety results of the 0.2 µg/day fluocinolone acetonide intravitreal implant for the treatment of diabetic macular oedema: the observational PALADIN study. Br J Ophthalmol. 2021;105(3):414-419. doi:10.1136/bjophthalmol-2020-315984
10. Luo C. Diabetic retinopathy progression in anti-VEGF vs. 0.19 mg fluocinolone acetonide implant treated eyes who are lost to follow up. Paper presented at: American Society of Retina Specialists 2020 Virtual Annual Meeting; July 24-26, 2020.
