Abstract
Acinic cell carcinoma (AiCC) in the nasal cavity and paranasal sinuses has rarely been reported in literature. A recent study demonstrated that recurrent genomic rearrangement [t(4;9) (q13;q31)] is a driver event in AiCC of the salivary glands that could promote the upregulation of transcription factor nuclear receptor subfamily 4 group A member 3 (NR4A3). In the current study, we evaluated the clinicopathological characteristics and expression of NR4A3 in four new cases of sinonasal AiCC. All four patients were men (range, 27–70 years). The tumor involved only the nasal cavity in two patients, while the other two patients showed involvement of both the nasal cavity and ethmoid sinus. Histologically, the tumor displayed a predominantly solid growth pattern and was composed of hematoxyphilic serous-like cells and scattered intercalated duct-like cells. Immunohistochemically, all cases expressed DOG-1. However, staining for mammaglobin, S-100, CA9, and P63 was absent in all patients. All four cases showed positive nuclear staining for NR4A3. In contrast, none of the other 39 sinonasal tumors, including secretory carcinomas, pleomorphic adenomas, mucoepidermoid carcinomas, adenoid cystic carcinomas, renal cell-like adenocarcinomas, intestinal-type adenocarcinomas, non-intestinal-type adenocarcinomas, extraskeletal myxoid chondrosarcoma, and carcinoma ex pleomorphic adenomas, presented with any positive NR4A3 nuclear staining. Additionally, NR4A3 rearrangements were observed in three cases with sinonasal AiCC by fluorescence in situ hybridization, and the expression level of NR4A3 mRNA was significantly increased in sinonasal AiCC compared with that in normal parotid tissue. Our study demonstrated that sinonasal AiCCs are characterized by an indolent nature and histopathological similarity to parotid AiCCs. Moreover, NR4A3 is a reliable biomarker for distinguishing sinonasal AiCCs from other sinonasal carcinomas.
Similar content being viewed by others
Introduction
Acinic cell carcinoma (AiCC) is a rare malignant salivary gland neoplasm characterized by acinar cells, according to the 4th edition of the World Health Organization classification of head and neck tumors1. Most AiCCs are localized in the parotid gland, whereas <10% of AiCCs arise outside nonparotid sites2. AiCCs occurring in the sinonasal cavity, in particular, are extremely rare, with few case reports3.
Recently, recurrent genomic rearrangement [t(4;9)(q13;q31)] was demonstrated to be a characteristic genetic driver in AiCCs of the salivary glands, which could promote upregulation of the transcription factor nuclear receptor subfamily 4 group A member 3 (NR4A3) through enhancer hijacking4. Breast AiCCs display several morphological similarities with their salivary gland counterparts. However, a previous study revealed that breast AiCCs harbored highly recurrent TP53 mutations and lacked any fusion genes, suggesting that breast AiCCs differ molecularly from salivary gland AiCCs5,6. Therefore, whether recurrent genomic rearrangement [t(4;9) (q13;q31)] is present in sinonasal AiCCs should be assessed. Haller et al.7 also demonstrated that NR4A3 immunostaining is a highly specific and sensitive novel marker for AiCCs of the salivary glands. However, the expression of NR4A3, as well as its diagnostic value in sinonasal AiCCs, remains uncertain. Additionally, the histopathological characteristics of sinonasal AiCC are poorly understood.
In this study, we reevaluated the histopathological features of sinonasal AiCCs. Furthermore, we investigated NR4A3 immunostaining and NR4A3 fluorescence in situ hybridization (FISH) in sinonasal AiCCs to validate the potential diagnostic utility of NR4A3 expression in sinonasal AiCCs.
Materials and methods
Patient cohort
In our cohort, we obtained formalin-fixed paraffin-embedded (FFPE) material from patients with an initial diagnosis of AiCC primarily arising from the sinonasal region (four cases) or parotid gland (ten cases) who underwent surgical resection at the Department of Otolaryngology of the Affiliated Eye Ear Nose and Throat (EENT) Hospital, Fudan University between October 2009 and August 2019. We also collected eight specimens from non-neoplastic salivary gland tissues to serve as controls. In addition, the medical records of sinonasal AiCC patients were reviewed to collect demographic and clinical features, including age, sex, tumor location, and clinical outcome. This study was approved by the Research and Ethics Committee of the EENT Hospital, affiliated with Fudan University.
In addition, NR4A3 immunostaining was also evaluated in patients with other histological sinonasal tumor subtypes, including two secretory carcinomas, five pleomorphic adenomas, five mucoepidermoid carcinomas, ten adenoid cystic carcinomas, three sinonasal renal cell-like adenocarcinomas, five intestinal-type adenocarcinomas, five non-intestinal-type adenocarcinomas (including two ETV6-rearranged low-grade cases), one extraskeletal myxoid chondrosarcoma, and three carcinomas ex pleomorphic adenomas. Two cases of ETV6-rearranged low-grade non-intestinal-type sinonasal adenocarcinomas in the cohort were reported in our previous study8. All diagnoses were confirmed by two experienced head and neck pathologists (C.W.Z. and L.L.).
Immunohistochemistry
The retrieved FFPE blocks were cut into 4-μm-thick sections. Immunohistochemistry (IHC) for most primary antibodies, including S-100 (4C4.9, 1:100 dilution; Gene Tech, Shanghai, China), SOX10 (EP268, prediluted, Maximum, Fuzhou, China), P63 (4A4, 1:100 dilution, Gene Tech, Shanghai), CA9 (H-11, prediluted, Gene Tech, Shanghai), DOG-1 (SP31, prediluted, Gene Tech, Shanghai), and mammaglobin (304-1A5, prediluted, Gene Tech, Shanghai), was performed using a BenchMark Autostainer (Ventana Medical Systems Inc., Tucson, AZ, USA) following the manufacturer’s protocol. For NR4A3 (clone H-7; Santa Cruz Biotechnology, Dallas, TX, USA) IHC staining, the sections were deparaffinized, subjected to antigen retrieval using 0.01 M citrate buffer at 95 °C for 5 min thrice and subsequently incubated for 15 min with 3% hydrogen peroxide to block endogenous peroxidase activity. The sections were incubated with 3% bovine serum albumin to block nonspecific staining. We used the same primary antibody for NR4A3 (also known as NOR-1), as reported in a previous study at a dilution of 1:509. Serial sections were incubated with primary antibodies in a humidified chamber at 4 °C overnight. The sections were then incubated with a horseradish peroxidase-conjugated secondary antibody (Gene Tech, Shanghai) for 30 min at room temperature. Diaminobenzidine (Gene Tech, Shanghai) was used as a chromogen, and the sections were counterstained with hematoxylin. The immunohistochemically stained sections were independently evaluated by two pathologists. The extent of immunohistochemical expression was quantified in quartiles as previously reported10: 0, negative; 1+, 1–25% positive cells; 2+, 26–50% positive cells; 3+, 51–75% positive cells; and 4+, 76–100% positive cells. Immunostaining intensity was recorded as weak, moderate, or strong.
Detection of NR4A3 by the FISH method
Four-micrometer-thick, formalin-fixed and paraffin-embedded tissue sections were used for FISH. As previously described, the presence of NR4A3 was assessed using the commercially available ZytoLight FISH Probe (ZytoLight SPEC NR4A3 Dual Color Break-Apart Probe, Z-2145-50; ZytoVision GmbH, Bremerhaven, Germany)7. For each slide, 50 randomly selected non-overlapping tumor cell nuclei were examined by a pathologist (C.Z.). Cells with two orange/green fusion signals were scored as normal. Separate green and orange signals in the nucleus were considered rearrangements of NR4A3. Tumors with >20% of cells exhibiting break-apart signals were considered positive.
RT-PCR
Total RNA was extracted from FFPE sections using the RNeasy FFPE kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The concentration and purity of the isolated RNA were assessed by optical density (OD) measurement using a Nanodrop spectrophotometer (BioTek, Winooski, VT, USA) after all extracted RNA was purified (OD = 1.8–2.0, 260/280 ratio). One percent agarose gel electrophoresis was used to assess the integrity of the RNA. RNA was then converted to complementary DNA (cDNA) using the SuperScript™ IV First-Strand Synthesis System (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The cDNA samples were subsequently subjected to a real-time polymerase chain reaction (RT-PCR) using an SYBR Green I Real-time system (Takara Bio Inc., Shiga, Japan) according to the manufacturer’s instructions. The primer sequences for the qualitative analyses were as follows4: GAPDH: forward, 5′-TGCACCACCAACTGCTTAGC-3′ and reverse, 5′-GGCATGGACTGTGGTCATGAG-3′; NR4A3: forward, 5′-CTCAACACCCAGAGATCTTGATTA-3′ and reverse, 5′-GTAGAATTGTTGCACATGCTCAG-3′. Expression levels were quantified using the 2-ΔΔCt method.
Statistical analysis
All data analyses were performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA, USA). Mann–Whitney U tests were used to assess the mRNA expression of NR4A3 in AiCCs and normal parotid tissue. A p value <0.05 was considered statistically significant.
Results
Clinical findings
The clinical features of the four cases are summarized in Table 1. All four patients were male, with ages ranging from 27 to 70 years at the time of surgery. All patients presented with persistent unilateral nasal obstruction, and two patients complained of epistaxis. The tumors were unilateral and involved only the nasal cavity in two patients, while the other two patients showed involvement of both the nasal cavity and ethmoid sinus. Regional or distant metastasis was absent in all four patients. None of the patients had a history of prior surgery or smoking. Endoscopic transnasal resection was performed for each patient, and one patient received postoperative radiotherapy. All four patients were alive without disease, with follow-up ranging from 19 to 96 months.
Histology and histopathology
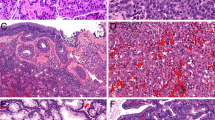
All specimens for pathological examination were fragmented and obtained from endoscopic surgical resection. Microscopically, the tumors were located beneath the epithelium and were non-encapsulated and well-circumscribed with an expansive growth pattern. All four cases uniformly displayed predominantly solid growth and appeared hematoxyphilic. Microcystic growth patterns could also be observed in several areas. The tumors were mainly composed of packed aggregates and differentiated serous-like cells (Fig. 1). A few intercalated duct-like cells were mixed with serous-like cells in all four cases. The serous-like cells contained hematoxyphilic zymogen granules and showed no significant cytological atypia or mitotic activity. The stroma component was less abundant and mainly composed of vasculature. No dedifferentiated or necrotic areas were observed in any of the four cases. Perineural or vascular invasion was absent in all four cases.
Table 2 summarizes the immunohistochemical results of the four patients with sinonasal AiCCs. Typically, moderate to strong and diffuse luminal DOG-1 expression was observed in three patients. SOX10 also showed positive expression in the tumor cells of these three patients (Fig. 2). However, the other patient (Case 1) had only focal and weak DOG-1 staining and negative SOX10 staining (Fig. 3). Negative staining for mammaglobin, S-100, CA9, and P63 was consistently observed in all patients (Fig. 2).
Immunohistochemical findings of NR4A3
In the eight normal parotid gland tissues, negative staining for NR4A3 was observed in the serous acinar cells. As a positive control, ten patients with salivary AiCCs showed strong and diffuse (3+ or 4+) nuclear staining of NR4A3. All four cases with sinonasal AiCCs displayed positive staining, including moderate 2+ staining in one case, strong 2+ staining in one case, and strong 4+ staining in two cases (Fig. 4). In contrast, none of the other 39 sinonasal tumors presented with any positive NR4A3 nuclear staining (Fig. 5 and Supplementary Fig. 1). Therefore, NR4A3 immunostaining had 100% sensitivity and 100% specificity for distinguishing sinonasal AiCCs from other sinonasal tumors.
Representative whole-mount sections of parotid AiCC (A) and Case 2 (D). High magnification shows both normal parotid gland tissue and tumor area in parotid AiCC (B). Sinonasal AiCC displays a solid growth pattern (E). Both parotid and sinonasal AiCCs showed identical strong nuclear NR4A3 staining (C, F).
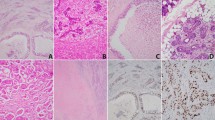
Negative staining was consistently seen in ETV6-rearranged low-grade non-intestinal-type sinonasal adenocarcinoma (A, B), intestinal-type adenocarcinoma (C, D), adenoid cystic carcinoma (E, F), mucoepidermoid carcinoma (G, H), carcinoma ex pleomorphic adenoma (I, J), pleomorphic adenoma (K–L), secretory carcinoma (M, N), and sinonasal renal cell-like adenocarcinomas (O, P).
Molecular findings
All four sinonasal AiCCs were further evaluated by NR4A3 FISH, and three cases tested positive for NR4A3 rearrangement (Fig. 6, Table 2). Additionally, we found that the NR4A3 mRNA level was significantly increased in tumor tissue from both salivary gland AiCC patients and sinonasal AiCC patients compared with that in normal parotid gland tissue (p < 0.001 and p < 0.01, respectively) (Fig. 7).
Discussion
Sinonasal malignancies comprise various histological subtypes and account for only 3–5% of all head and neck cancers11. Primary sinonasal AiCCs are extremely rare, with few case reports in literature12. Since the initial description of secretory carcinoma as a distinctive salivary gland neoplasm in 2010 by Skalova et al. subsequent studies found that a high proportion of nonparotid AiCC was misdiagnosed and should be reclassified as secretory carcinoma13,14,15. Moreover, although secretory carcinomas in the parotid share similarities in terms of their biological aggressiveness and prognosis, the accurate identification of secretory carcinoma has the potential benefit of targeted therapy using NTRK inhibitors16. In the present study, we comprehensively reviewed the clinicopathological and molecular features of sinonasal AiCCs. To the best of our knowledge, this is the first study to reveal that NR4A3, a newly described marker, could also serve as a reliable marker to distinguish sinonasal AiCCs from other sinonasal tumors.
In 2014, Biron et al.3 performed the largest retrospective analysis of clinical features and outcomes in 18 cases of sinonasal AiCCs using the Surveillance, Epidemiology, and End Results database over 30 years. In line with this previous report, we found that most cases occurred in the fifth and sixth decades of life. The symptoms presented in our series were nonspecific. Consistent with Biron’s report, all four patients showed limited extension without any nodal or distant metastases. None of the patients developed recurrence in our series, and the estimated 10-year recurrence-free survival was 92.9% in a previous meta-analysis. The low recurrence risk was likely related to the low histological grade and limited tumor involvement. Therefore, sinonasal AiCC is characterized by an indolent growth pattern and favorable outcomes. Due to its limited extension, endoscopic surgery could serve as the primary treatment for sinonasal AiCCs. Only one patient in our series received postoperative radiotherapy. In Biron’s report, 18.7% of the patients received postoperative radiation. Although the clear indications of radiotherapy have not been clarified for sinonasal AiCCs, patients with positive surgical margins would probably benefit from adjuvant radiotherapy.
In accordance with a previous report, sinonasal AiCCs share similar histopathological features with parotid AiCCs, which are also characterized by predominantly serous cell components containing zymogen granules admixed with intercalated duct-like components12. However, no areas of papillary or follicular growth were observed in our four cases. Additionally, none of the four cases harbored high-grade transformation, which may also explain their favorable prognosis. The immunohistochemical profiles of sinonasal AiCC were almost identical to those of parotid AiCC, presenting with negative staining for P63, S-100, and mammaglobin and positivity for DOG-1. Despite several overlapping histological features such as serous differentiation, the tinctorial properties of the secretory granules in salivary gland as well as sinonasal AiCCs are distinct from breast AiCCs, with predominantly hematoxyphilic granules in the former lesions and eosinophilic granules in the latter. Moreover, the typical infiltrative microglandular growth pattern in breast AiCCs is rarely seen in salivary gland AiCCs and sinonasal AiCCs17. Although DOG-1 has been proposed as a useful biomarker to distinguish sinonasal AiCCs from secretory carcinoma, positive staining for DOG-1 could also be seen in other salivary carcinomas, including adenoid cystic carcinoma18. Moreover, a newly described entity termed ETV6-rearranged low-grade non-intestinal-type sinonasal adenocarcinoma also showed positive staining for DOG-1 on the luminal membrane19. In the current study, we found one case that presented with only very weak and focal membranous staining for DOG-1. Hence, a more reliable biomarker is required for the differential diagnosis of sinonasal AiCC.
Consistent with a previous study, we observed positive staining of NR4A3 in all cases with parotid AiCC and no positive staining in normal parotid tissue7. In addition to the overlapping histological features of sinonasal and parotid AiCCs, sinonasal AiCCs also displayed consistently positive nuclear expression of NR4A3 as parotid AiCCs. Overexpression of NR4A3 in mouse salivary gland cells has been suggested to promote downstream gene expression, including the cell regulator cyclin D1, which is associated with cell proliferation4. Therefore, this implies the potential value of NR4A3 in the differential diagnosis of sinonasal AiCCs from other sinonasal malignancies, as well as its effect on pathogenesis. Previously, the overexpression of NR4A3 protein was shown to be associated with transcriptional activation of gene expression4,20. In the current study, elevated NR4A3 mRNA was detected in all AiCC patients, which was correlated with positivity for NR4A3 by IHC.
Previously, Haller et al.4 reported that parotid AiCCs harbored recurrent genomic rearrangement [t(4;9)(q13;q31)], leading to the upregulation of NR4A3 via “enhancer hijacking.” Recently, Lee et al.21 identified new recurrent translocations, including t(9;12), t(8;9), or t(2;4) chromosomal translocations, which are also located in the upstream region of the NR4A3 gene or the closely related NR4A2 gene. In the current study, we found split signals in three cases with sinonasal AiCCs by commercial NR4A3 break-apart FISH, which implied the presence of genomic rearrangements of the NR4A3 gene locus. Another case with a normal FISH signal but positive NR4A3 nuclear staining may have been related to the 9q31 breakpoint far upstream of the NR4A3 gene locus, as suggested by Haller et al.7. However, the precise breakpoint in our four sinonasal AiCCs needs to be clarified by next-generation sequencing. In line with previous reports, our data suggest that NR4A3 immunostaining would be more sensitive than FISH for identifying sinonasal AiCC. Additionally, sinonasal AiCCs presented with identical histopathological features and molecular backgrounds as those of salivary gland AiCCs.
Although sinonasal AiCCs are mostly characterized by conventional solid growth patterns with serous-like cells, which makes morphologic diagnosis straightforward, the low incidence and small fragmented biopsy would also pose significant challenges in identifying the entity by pathologists. In particular, previous studies have suggested that most nonparotid AiCCs with absent serous acinar differentiation should be reclassified as (mammary analog) secretory carcinomas13,14. The ETV6-rearranged low-grade sinonasal non-intestinal-type adenocarcinomas also show several overlapping morphological features with sinonasal AiCCs, including bland tumor cells with rare mitotic activity and DOG-1 positivity19. Therefore, specific biomarkers for IHC would aid pathologists in the differential diagnosis of sinonasal AiCCs. Recent studies have found that NR4A3 immunostaining is a highly specific marker and more sensitive than DOG-1 immunostaining for diagnosing AiCCs of the salivary gland, even using fine-needle aspiration biopsy specimens22,23,24. In the current study, we first evaluated the diagnostic value of NR4A3 in the differential diagnosis of sinonasal AiCCs. We observed complete negative staining of NR4A3 in other sinonasal tumors, including sinonasal secretory carcinoma and ETV6-rearranged low-grade non-intestinal-type adenocarcinomas. Although NR4A3 gene rearrangements are also characteristic of extraskeletal myxoid chondrosarcoma, NR4A3 immunostaining was negative in this tumor type25. Our results indicate that NR4A3 is a specific biomarker for sinonasal AiCCs. Furthermore, NR4A3 showed clear nuclear staining in our four cases, whereas weak and focal membranous staining of DOG-1 was observed in one patient. Therefore, NR4A3 was more reliable and easier to interpret than DOG-1 for the diagnosis of sinonasal AiCCs.
The main limitation of this study is that the sample sizes of both sinonasal AiCCs and other types of sinonasal carcinomas were small. Therefore, the potential value of NR4A3 in differential diagnosis should be further confirmed. Additionally, a small subset of polymorphous adenocarcinomas (15%) was found to have focally positive staining for NR4A39. Polymorphous adenocarcinomas always occur at the junction of hard and soft palates and are extremely rare in the sinonasal region. We reviewed the pathology archive in our hospital and did not find any cases of sinonasal polymorphous adenocarcinomas in the past 10 years. Hence, NR4A3 immunostaining in sinonasal polymorphous adenocarcinomas remains to be further evaluated. However, due to the low proportion and relatively weak intensity of NR4A3 immunostaining in polymorphous adenocarcinomas, we believe that NR4A3 remains a highly specific marker for sinonasal AiCCs.
In summary, we demonstrated that sinonasal AiCC is an indolent subtype of sinonasal carcinoma with pathological and molecular features similar to those of its parotid counterparts. Therefore, NR4A3 should be added to the immunohistochemical detection panel, and can be used for the diagnosis and differential diagnosis of sinonasal AiCC.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information Files.
References
El-Naggar, A. K., C. J., Grandis, J. R., Takata, T. & Slootweg, P. J. WHO Classification of Head and Neck Tumours. (IARC, 2017).
Vander Poorten, V. et al. Salivary acinic cell carcinoma: reappraisal and update. Eur. Arch. Otorhinolaryngol. 273, 3511–3531 (2016).
Biron, V. L., Lentsch, E. J., Gerry, D. R. & Bewley, A. F. Case-control analysis of survival outcomes in sinonasal acinic cell carcinoma. Int. Forum Allergy Rhinol. 4, 507–511 (2014).
Haller, F. et al. Enhancer hijacking activates oncogenic transcription factor NR4A3 in acinic cell carcinomas of the salivary glands. Nat. Commun. 10, 368 (2019).
Piscuoglio, S. et al. Are acinic cell carcinomas of the breast and salivary glands distinct diseases? Histopathology 67, 529–537 (2015).
Beca, F. et al. Whole-exome sequencing and RNA sequencing analyses of acinic cell carcinomas of the breast. Histopathology 75, 931–937 (2019).
Haller, F. et al. Nuclear NR4A3 immunostaining is a specific and sensitive novel marker for acinic cell carcinoma of the salivary glands. Am. J. Surg. Pathol. 43, 1264–1272 (2019).
Zhai, C. W., Yuan, C. C. & Wang, S. Y. ETV6-rearranged low-grade sinonasal non-intestinal-type adenocarcinoma: a clinicopathological analysis. Zhonghua Bing Li Xue Za Zhi 50, 55–59 (2021).
Wong, K. S., Marino-Enriquez, A., Hornick, J. L. & Jo, V. Y. NR4A3 immunohistochemistry reliably discriminates acinic cell carcinoma from mimics. Head Neck Pathol. 15, 425–432 (2021).
Zhai, C., Wang, H., Li, S. & Wang, D. Clinicopathological analysis of low-grade papillary Schneiderian carcinoma: report of five new cases and review of the literature. Histopathology 79, 370–380 (2021).
Hermsen, M. A. et al. Translational genomics of sinonasal cancers. Semin. Cancer Biol 61, 101–109 (2020).
Neto, A. G., Pineda-Daboin, K., Spencer, M. L. & Luna, M. A. Sinonasal acinic cell carcinoma: a clinicopathologic study of four cases. Head Neck 27, 603–607 (2005).
Chiosea, S. I., Griffith, C., Assaad, A. & Seethala, R. R. The profile of acinic cell carcinoma after recognition of mammary analog secretory carcinoma. Am. J. Surg. Pathol. 36, 343–350 (2012).
Bishop, J. A., Yonescu, R., Batista, D., Eisele, D. W. & Westra, W. H. Most nonparotid “acinic cell carcinomas” represent mammary analog secretory carcinomas. Am. J. Surg. Pathol. 37, 1053–1057 (2013).
Skalova, A. et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am. J. Surg. Pathol. 34, 599–608 (2010).
Terada, T. et al. Clinical characteristics of acinic cell carcinoma and secretory carcinoma of the parotid gland. Eur. Arch. Otorhinolaryngol. 276, 3461–3466 (2019).
Pareja, F., Weigelt, B. & Reis-Filho, J. S. Problematic breast tumors reassessed in light of novel molecular data. Mod. Pathol. 34, 38–47 (2021).
Khurram, S. A. & Speight, P. M. Characterisation of DOG-1 expression in salivary gland tumours and comparison with myoepithelial markers. Head Neck Pathol. 13, 140–148 (2019).
Andreasen, S. et al. ETV6 gene rearrangements characterize a morphologically distinct subset of sinonasal low-grade non-intestinal-type adenocarcinoma: a novel translocation-associated carcinoma restricted to the sinonasal tract. Am. J. Surg. Pathol. 41, 1552–1560 (2017).
Haller, F. et al. Nuclear NR4A2 (Nurr1) immunostaining is a novel marker for acinic cell carcinoma of the salivary glands lacking the classic NR4A3 (NOR-1) upregulation. Am. J. Surg. Pathol. 44, 1290–1292 (2020).
Lee, D. Y. et al. Oncogenic orphan nuclear receptor NR4A3 interacts and cooperates with MYB in acinic cell carcinoma. Cancers 12 (2020).
Nguyen, L. et al. NOR-1 distinguishes acinic cell carcinoma from its mimics on fine-needle aspiration biopsy specimens. Hum. Pathol. 102, 1–6 (2020).
Skaugen, J. M., Seethala, R. R., Chiosea, S. I. & Landau, M. S. Evaluation of NR4A3 immunohistochemistry (IHC) and fluorescence in situ hybridization and comparison with DOG1 IHC for FNA diagnosis of acinic cell carcinoma. Cancer Cytopathol. 129, 104–113 (2021).
Owosho, A., Tyler, D., Adesina, O., Odujoko, O. & Summersgill, K. NR4A3 (NOR-1) immunostaining shows better performance than DOG1 immunostaining in acinic cell carcinoma of salivary gland: a preliminary study. J. Oral Maxillofac. Res. 12, e4 (2021).
Vargas, A. C., Maclean, F. M., Bonar, F., Mahar, A. & Gill, A. J. NR4A3 immunohistochemistry lacks sensitivity for the diagnosis of extraskeletal myxoid chondrosarcoma. Am. J. Surg. Pathol. 43, 1726–1728 (2019).
Acknowledgements
This work was supported by Natural Science Foundation of China (no. 82000953), the Clinical Research Plan of SHDC (no. SHDC2020CR2005A), Chinese Academy of Medical Sciences Foundation “Research Units of New Technologies of Endoscopic Surgery in Skull Base Tumor(2018RU003) and Shanghai Science and Technology Committee Foundation (19411950600).
Funding
This work was supported by Natural Science Foundation of China (no. 82000953), the Clinical Research Plan of SHDC (no. SHDC2020CR2005A), Chinese Academy of Medical Sciences Foundation “Research Units of New Technologies of Endoscopic Surgery in Skull Base Tumor(2018RU003) and Shanghai Science and Technology Committee Foundation (19411950600).
Author information
Authors and Affiliations
Contributions
H.W., C.Z. and C.Z. performed the experiments and measurements, H.W., Q.L. and H.Z. processed the experimental data, performed the analysis, drafted the paper, and designed the figures. X.S. aided in interpreting the results and worked on the paper. L.L., H.Y. and D.W. were involved in planning and supervising the work and also the final approval of the paper. All authors discussed the results and commented on the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the ethics committee of Eye, Ear, Nose and Throat Hospital affiliated to Fudan University, Shanghai, China. All authors agree to participate this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, H., Zhai, C., Zhang, C. et al. Analysis of clinicopathologic features and expression of NR4A3 in sinonasal acinic cell carcinoma. Mod Pathol 35, 594–600 (2022). https://doi.org/10.1038/s41379-021-00959-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00959-8