Schuster Reviews Potential Therapeutic Options in Second-Line DLBCL
Following 6 well-tolerated cycles of R-CHOP, a 75-year-old patient with diffuse large B-cell lymphoma showed complete remission on a PET scan. But 1 year later, the disease relapsed.
Stephen J. Schuster, MD

During a Targeted OncologyTM Case-Based Roundtable event, Stephen J. Schuster, MD, Robert and Margarita Louis-Dreyfus professor in Chronic Lymphocytic Leukemia and Lymphoma Clinical Care and Research, Division of Hematology Oncology, Perelman Center for Advanced Medicine, Hospital of the University of Pennsylvania, led a discussion about a 75-year-old patient with diffuse large B-cell lymphoma.
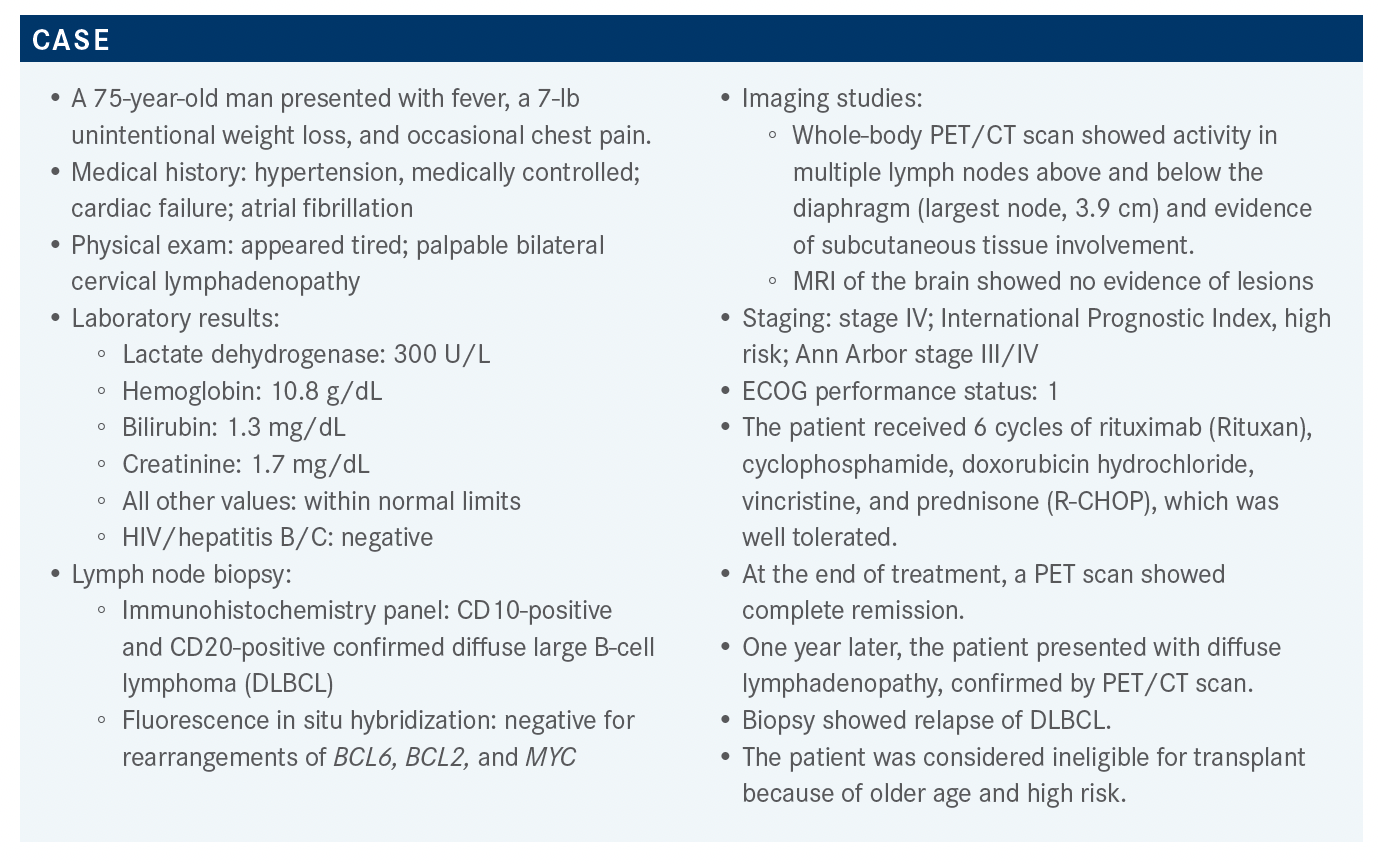
Targeted OncologyTM: Outside of a clinical trial, what treatment options are there for this patient?
SCHUSTER: If you’re going to use chimeric antigen receptor [CAR] T-cell therapy, you have to have a strategy, because until we have data from a randomized trial you won’t to be able to get coverage for this therapy, which is expensive. Low-intensity chemotherapy is not unreasonable, particularly if it’s a bridge to CAR T-cell therapy. I don’t think that high-intensity chemotherapy is a great option. [Regarding] patients in the rituximab era that relapsed at this time, even the data from the CORAL trial [NCT00137995] showed only about 20% of them are long-term survivors.1
The National Comprehensive Cancer Network [NCCN] guidelines [for second-line and subsequent therapies in transplant-ineligible patients]2 include [a regimen] approved by the FDA, polatuzumab vedotin [Polivy] and bendamustine, to be used after 2 or more prior regimens.3 [Regimens listed as] “useful in certain circumstances” include lenalidomide [Revlimid] with or without rituximab and ibrutinib [Imbruvica], which has about a 30% to 40% chance of response in the activated B-cell type [disease].4 [Of all the regimens listed], the only 1 that is approved for patients who have received 1 line of therapy [and are ineligible for transplant] is tafasitamab-cxix [Monjuvi] plus lenalidomide,5 but the other regimens are [considered] reasonable to use. The third-line regimens [include the] FDA-approved CAR T-cell [therapies] loncastuximab tesirine [Zynlonta] and selinexor [Xpovio].2
What is your experience with bendamustine plus rituximab in patients with DLBCL?
In my experience, that combination doesn’t cure anybody. [It does shrink tumors], but you can get better results if you add polatuzumab.6 What I don’t like about that combination is that bendamustine is one of the best lympho-depleting agents we have. [This is a problem] if, later, you want to use CAR T-cell therapy or a therapy like tafasitamab plus lenalidomide that relies on healthy natural killer [NK] cells. Bendamustine destroys T cells and NK cells for 6 months or more, and you’ll likely lose your opportunity for pheresis [because most] manufacturers want a fresh product. Unless you can do pheresis before giving bendamustine, I’d avoid bendamustine because it is not successful in enough patients.
What second-line regimen would you recommend for this patient at the present time?
I would probably use tafasitamab and lenalidomide. I would assume this patient is chemotherapy resistant because he progressed within a year of R-CHOP therapy. That’s a [strong sign that chemotherapy will not be helpful for long]. Tafasitamab⁷ and lenalidomide⁸ are totally biologic.
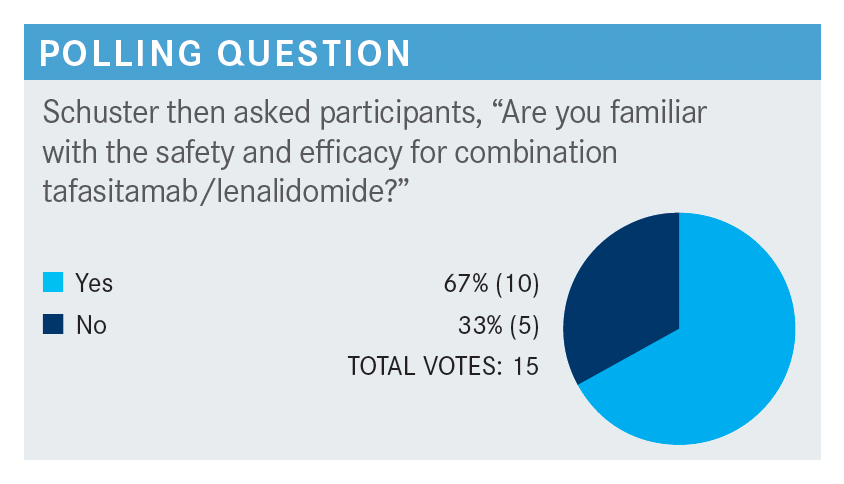
How do you feel about using tafasitamab and lenalidomide in this setting?
[In my experience], the best results [with this therapy] are achieved in the second line. It’s a reasonable second-line therapy for a patient who is not eligible for transplant, who refuses transplant, or who relapses within 12 months.
The mechanism of action of lenalidomide [is to] increase NK cell activity and enhance antibody-dependent cellular cytotoxicity.8 The antibody that is [administered] with it, tafasitamab, formerly MOR208, has been engineered in the Fc portion to minimize complement-mediated cytotoxicity and enhance the antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis functions of the Fc receptor. [That is], it was engineered to react more avidly with the Fcγ receptor on NK cells and macrophages.7,9-11
Which trial supports the use of the tafasitamab plus lenalidomide combination in DLBCL?
This combination was approved as a result of the L-MIND phase 2 trial [NCT02399085]. The FDA [will often grant approval based on phase 2 data] if a regimen addresses an unmet medical need, as long as there is a promise to collect additional data once the regimen gets to the market. [This trial involved mostly] patients who had received 1 or 2 prior lines of therapy; a few had received 3 prior lines of therapy.10-12
In this trial, patients’ median age was 72; International Prognostic Index scores were evenly distributed, as were the number of patients who were refractory to their last prior therapy. From among 81 patients, 19 patients had primary refractory disease using a stringent diagnosis limit of 6 months. There were not enough data to conclude whether [cell of origin influenced the results]. Finally, some patients, but not many, had [had] a transplant.12
The patient characteristics [are similar to those in] other studies, with the exception of [the number of] prior lines of therapy. Half of these patients had failed only R-CHOP [and] almost all the other patients had failed 2 lines. Very few patients had failed 3 or 4 lines.12 If you want to [apply] the results of this study [to your practice], I favor using this regimen in the second line.
What was the efficacy of this combination in the L-MIND trial?
From among all patients, including those who required a dose reduction, 43% achieved a complete response [(CR) after a median follow-up of 13.2 months].12 Three years later, 40% of the patients were still in CR.13,14 These results are [comparable to those seen with] CAR T-cell therapy but in a different population, because these were mostly second-line patients. [To receive] CAR T-cell therapy, patients have to be in the third line or later, [and we see no more than 40% of CAR T-cell patients] in remission beyond 3 years.
The partial response [PR] rates were [18% after a median follow-up of 13.2 months12 and 17.5% after a median follow-up of ≥ 35 months].13,14 The overall response rates were very good [at 60% and 57.5% at the same respective time points].12-14 The [best result] was the CR. Disease control is important. The longer you can go without giving somebody chemotherapy, the better.
At about 12 months, median progression-free survival [PFS] was reached.12 At approximately 3 years’ follow-up, median PFS was [similar], 11.6 months.13 At about 4 years of follow-up, one-third of the patients were alive [median overall survival, 33.5 months], which is not bad for people who are progressing on second-line therapy.
The median duration of response [DOR] for patients with CR hasn’t been reached at almost 6 years of follow-up [95% CI, 43.9 months-not reached (NR)]. Patients with a PR, of course, did worse [median DOR, 5.6 months; 95% CI, 2.2 months-NR].
Efficacy was analyzed according to the number of prior lines of therapy. Among patients who had received 1 prior line of therapy, the rate of patients with a best objective response of CR was [about] 47%, or 19 of 40 evaluable patients [vs about 32% among those who had received 2 or more prior lines]. I don’t know if [the latter group had a worse response] because the disease was more resistant or because previous treatments like bendamustine and cytotoxic agents [impeded] the cell-mediated cytotoxicity that tafasitamab [is meant to enhance]. It was probably both.
I think this regimen will turn out to be a good second-line regimen, particularly in chemotherapy-resistant patients. Instead of [wearing down the patient and destroying their] T cells with other chemotherapy, I like to use this; it spares the T cells. This is particularly [good for] patients who progress early and [therefore] haven’t had time for full T-cell recovery.
Can tafasitamab be used as a single agent to avoid the adverse events of lenalidomide?
A post hoc analysis was done to see whether PFS was affected by discontinuation of lenalidomide, and the PFS curve was [unchanged (median PFS, 12.7 months)].15 So tafasitamab does have some single-agent activity, but the best activity is [achieved when it is used] with lenalidomide.9,15
However, a significant percentage of patients [in this trial] couldn’t tolerate the lenalidomide. Almost a quarter…had to have a dose reduction below 20 mg of lenalidomide [and nearly] a quarter…had to have permanent discontinuation. So almost half the patients received either a dose reduction or elimination of lenalidomide. There were only 62 patients, about three-fourths, who could maintain a lenalidomide dose of 20 mg or more.12
In my experience, very few patients with lymphoma can tolerate 25 mg of lenalidomide for 3 weeks on and 1 week off. I think you [should] start according to protocol and then follow the protocol if you need to do a dose reduction.12
What treatment-emergent adverse events [TEAEs] were reported in this study?
The most common hematologic TEAEs were cytopenias, primarily neutropenia [of grade 4, affecting 21% of patients].7,12 Those who are familiar with lenalidomide are used to this. Other clinically relevant TEAEs included fatigue and diarrhea, [both commonly seen with lenalidomide treatment]. Some of the other TEAEs were those commonly seen in patients whose therapy is not working.
Among serious AEs, those suspected to be treatment-related affected 15 of 81 enrolled patients [19%]. Additionally, 10 of 81 discontinued therapy [12%]. AEs of special interest [affecting 9% of patients] included things like tumor flare.7,12,13
Safety was analyzed by treatment phase. Most of the AEs [occurred during the first phase], when patients were receiving the tafasitamab plus lenalidomide combination [vs during the second, tafasitamab-monotherapy phase],10 and so the AEs were probably attributable to the combination. The safety profile is not very different from what I’ve [observed when] using lenalidomide plus rituximab in similar patients.
Are there data to support the use of this combination in patients with c-MYC translocations?
There were 7 patients in the original trial who had c-MYC translocations. One had a double hit, and that patient’s PR only lasted 6 months. A patient with a triple hit had a CR and was still in remission at the 20-month follow-up.13
So, like CAR T-cell therapy, [this regimen] seems to have some activity in patients with double and triple hits. [But keep in mind], there were only 7 patients with c-MYC translocations, and among them, 3 achieved CR, 1 achieved PR, and 3 patients had no response. Why were there so
few c-MYC rearrangements [represented] in this study? Because the population consisted of people who had relapsed only once.12 [One would be more likely to find] c-MYC rearrangements, double hits, and triple hits among patients who had relapsed after multiple regimens.
Can tafasitamab be continued forever if a patient achieves CR?
I would say that for most aggressive lymphomas, if a patient is still in remission 2 years [after frontline therapy], they are about 80% [safe from relapse]. If they are in remission at 5 years, the likelihood that they are going to relapse gets extremely low regardless of therapy.
Between 2 and 5 years, you have to use your judgment. I’m not convinced that doing things like a molecular minimal residual disease assay is going to help. I think PET scans can guide you. If a patient is in a durable [remission as assayed by] PET/CT scan beyond a couple of years, you may want to consider discontinuing therapy.
If this regimen were to fail in the second line, should CAR T-cell therapy be offered, given that both are CD19-targeted strategies?
We don’t know the answer to that question, but in other CD19-targeting strategies, it works. [For example], some patients have received CAR T-cell therapy following loncastuximab or tafasitamab, with success. I think the reason is that patients with lymphoma very rarely lose CD19 expression. The JULIET trial [NCT02445248] showed that the amount of CD19 expression, [assayed immunohistochemically], did not correlate with response [to tisagenlecleucel].16
In our own study, which [assayed CD19 expression by] flow cytometry, 10% or fewer patients have [decreased expression] or loss of CD19 when they relapsed following CAR T-cell therapy. So having CD19 is not a guarantee that [CAR T-cell therapy] is going to work, and [lack of CD19 expression], when assayed by immunohistochemistry, had no statistically significant effects in the JULIET trial.16
It’s good to be concerned. If you’re worried, you could do a fine-needle aspiration; more than 90% of the time, CD19 will be [observable] by flow cytometry. I think patients are in worse shape if [they’re receiving] chemotherapy because that depletes the T cells they need for CAR T-cell therapy. Lenalidomide does not deplete T cells; I like that about this regimen and I think it’s a reasonable strategy. I don’t feel we have data that suggest that [using this regimen precludes subsequent CAR T-cell therapy].
References:
1. Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184-4190. doi:10.1200/JCO.2010.28.1618
2. NCCN. Clinical Practice Guidelines in Oncology. B-cell lymphomas, version 5.2021. Accessed October 25, 2021. https://bit.ly/3C6hvVg
3. FDA approves polatuzumab vedotin-piiq for diffuse large B-cell lymphoma. FDA. June 10, 2019. Accessed October 26, 2021. https://bit.ly/3n5DJCw
4. Goy A, Ramchandren R, Ghosh N, et al. Ibrutinib plus lenalidomide and rituximab has promising activity in relapsed/refractory non-germinal center B-cell-like DLBCL. Blood. 2019;134(13):1024-1036. doi:10.1182/blood.2018891598
5. FDA grants accelerated approval to tafasitamab-cxix for diffuse large B-cell lymphoma. FDA. Updated August 3, 2020. Accessed October 25, 2021. https:// bit.ly/3kqlupE
6. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38(2):155-165. doi:10.1200/JCO.19.00172
7. Monjuvi. Prescribing information. MorphoSys US Inc; 2020. Accessed October 26, 2021. https://bit.ly/3qrBb46
8. Revlimid. Prescribing information. Celgene Corporation; 2021. Accessed October 26, 2021. https://bit.ly/3C8BHWG
9. Jurczak W, Zinzani PL, Hess G, et al. A phase IIa, open-label, multicenter study of single-agent tafasitamab (MOR208), an Fc-optimized anti- CD19 antibody, in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma: long-term follow-up, final analysis. Blood. 2019;134(suppl 1):4078. doi:10.1182/blood-2019-124297
10. Salles G, Duell J, González Barca E, et al. Primary analysis results of the single-arm phase II study of MOR208 plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (L-MIND). Paper presented at: 15th International Conference on Malignant Lymphoma; June 18-22, 2019; Lugano, Switzerland. Accessed October 25, 2021. https://bit.ly/3bXUGZo
11. Salles G, Duell J, González Barca E, et al. Primary analysis results of the single-arm phase II study of MOR208 plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma (L-MIND). Hematol Oncol. 2019;37(suppl 2):173-174. doi:10.1002/hon.130_2629
12. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. doi:10.1016/S1470-2045(20)30225-4
13. Düll J, Maddocks KJ, González-Barca E, et al. Long-term analyses from L-MIND, a phase II study of tafasitamab (MOR208) combined with lenalidomide (LEN) in patients with relapsed or refractory diffuse large B-cell lymphoma (R/R DLBCL). Paper presented at: 2021 American Society of Clinical Oncology Annual Meeting; June 4-8, 2021; Chicago, IL. Accessed October 27, 2021. https://bit. ly/30a8QUP
13. Duell J, Maddocks KJ, González-Barca E, et al. Long-term outcomes from the phase II L-MIND study of tafasitamab (MOR208) plus lenalidomide in patients with relapsed or refractory diffuse large B-cell lymphoma. Haematologica. 2021;106(9):2417-2426. doi:10.3324/haematol.2020.275958
14. Düll J, Topp M, Salles G. The use of tafasitamab in diffuse large B-cell lymphoma. Ther Adv Hematol. Published July 6, 2021. doi:10.1177/20406207211027458
15. Schuster SJ, Bishop MR, Tam CS, et al; JULIET Investigators. Tisagenlecleucel in adult relapsed or refractory diffuse large b-cell lymphoma. N Engl J Med. 2019;380(1):45-56. doi:10.1056/NEJMoa1804980

Challenges for Non–CAR T-Cell Treatment of Early Relapsed DLBCL
April 18th 2024During a Case-Based Roundtable® event, Elizabeth A. Brém, MD, discussed treatment approaches for a patient with early relapsed or primary refractory diffuse large B-cell lymphoma in the first article of a 2-part series.
Read More
Powell Reviews Updated IO/TKI Data and AE Management in Endometrial Cancer
April 18th 2024During a Case-Based Roundtable® event, Matthew A. Powell, MD, discussed the case of a patient with advanced endometrial cancer treated with lenvatinib plus pembrolizumab who experienced grade 2 treatment-related hypertension.
Read More