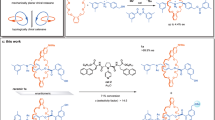
Abstract
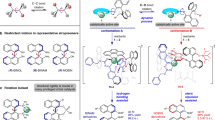
Rotaxanes can display molecular chirality solely due to the mechanical bond between the axle and encircling macrocycle without the presence of covalent stereogenic units. However, the synthesis of such molecules remains challenging. We have discovered a combination of reaction partners that function as a chiral interlocking auxiliary to both orientate a macrocycle and, effectively, load it onto a new axle. Here we use these substrates to demonstrate the potential of a chiral interlocking auxiliary strategy for the synthesis of mechanically planar chiral rotaxanes by producing a range of examples with high enantiopurity (93–99% e.e.), including so-called ‘impossible’ rotaxanes whose axles lack any functional groups that would allow their direct synthesis by other means. Intriguingly, by varying the order of bond-forming steps, we can effectively choose which end of an axle the macrocycle is loaded onto, enabling the synthesis of both hands of a single target using the same reactions and building blocks.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2069804 (rac-4b(1)) and 2093365 (rac-4b(2)). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
Characterization data (NMR, mass spectrometry, circular dichroism spectroscopy, HPLC) for all novel compounds reported here are available from the University of Southampton repository at https://doi.org/10.5258/SOTON/D1935
Change history
17 December 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41557-021-00875-z
References
Bruns, C. J. & Stoddart, J. F. The Nature of the Mechanical Bond: From Molecules to Machines (Wiley, 2016).
Erbas-Cakmak, S., Leigh, D. A., McTernan, C. T. & Nussbaumer, A. L. Artificial molecular machines. Chem. Rev. 115, 10081–10206 (2015).
Heard, A. W. & Goldup, S. M. Simplicity in the design, operation, and applications of mechanically interlocked molecular machines. ACS Cent. Sci. 6, 117–128 (2020).
Jamieson, E. M. G., Modicom, F. & Goldup, S. M. Chirality in rotaxanes and catenanes. Chem. Soc. Rev. 47, 5266–5311 (2018).
Evans, N. H. Chiral catenanes and rotaxanes: fundamentals and emerging applications. Chem. Eur. J. 24, 3101–3112 (2018).
Nakazono, K. & Takata, T. Mechanical chirality of rotaxanes: synthesis and function. Symmetry 12, 144 (2020).
Maynard, J. R. J. & Goldup, S. M. Strategies for the synthesis of enantiopure mechanically chiral molecules. Chem 6, 1914–1932 (2020).
Kaida, Y., Okamoto, Y., Chambron, J. C., Mitchell, D. K. & Sauvage, J. P. The separation of optically-active copper (i) catenates. Tetrahedron Lett. 34, 1019–1022 (1993).
Yamamoto, C., Okamoto, Y., Schmidt, T., Jager, R. & Vogtle, F. Enantiomeric resolution of cycloenantiomeric rotaxane, topologically chiral catenane, and pretzel-shaped molecules: observation of pronounced circular dichroism. J. Am. Chem. Soc. 119, 10547–10548 (1997).
Hirose, K. et al. The asymmetry is derived from mechanical interlocking of achiral axle and achiral ring components—syntheses and properties of optically pure [2]rotaxanes. Symmetry 10, 20 (2018).
Makita, Y. et al. Catalytic asymmetric synthesis and optical resolution of planar chiral rotaxane. Chem. Lett. 36, 162–163 (2007).
Tian, C., Fielden, S. D. P., Perez-Saavedra, B., Vitorica-Yrezabal, I. J. & Leigh, D. A. Single-step enantioselective synthesis of mechanically planar chiral [2]rotaxanes using a chiral leaving group strategy. J. Am. Chem. Soc. 142, 9803–9808 (2020).
Denis, M. & Goldup, S. M. The active template approach to interlocked molecules. Nat. Rev. Chem. 1, 0061 (2017).
Tian, C., Fielden, S. D. P., Whitehead, G. F. S., Vitorica-Yrezabal, I. J. & Leigh, D. A. Weak functional group interactions revealed through metal-free active template rotaxane synthesis. Nat. Commun. 11, 744 (2020).
Heard, A. W. & Goldup, S. M. Synthesis of a mechanically planar chiral rotaxane ligand for enantioselective catalysis. Chem 6, 994–1006 (2020).
Gaedke, M. et al. Chiroptical inversion of a planar chiral redox-switchable rotaxane. Chem. Sci. 10, 10003–10009 (2019).
Ishiwari, F., Nakazono, K., Koyama, Y. & Takata, T. Induction of single-handed helicity of polyacetylenes using mechanically chiral rotaxanes as chiral sources. Angew. Chem. Int. Ed. 56, 14858–14862 (2017).
Imayoshi, A. et al. Enantioselective preparation of mechanically planar chiral rotaxanes by kinetic resolution strategy. Nat. Commun. 12, 404 (2021).
Eliel, E., Wilen, S. & Mander, L. Stereochemistry of Organic Compounds (Wiley, 1994).
Jinks, M. A. et al. Stereoselective synthesis of mechanically planar chiral rotaxanes. Angew. Chem. Int. Ed. 57, 14806–14810 (2018).
Denis, M., Lewis, J. E. M., Modicom, F. & Goldup, S. M. An auxiliary approach for the stereoselective synthesis of topologically chiral catenanes. Chem 5, 1512–1520 (2019).
Aucagne, V., Hanni, K. D., Leigh, D. A., Lusby, P. J. & Walker, D. B. Catalytic “click” rotaxanes: a substoichiometric metal-template pathway to mechanically interlocked architectures. J. Am. Chem. Soc. 128, 2186–2187 (2006).
Lahlali, H., Jobe, K., Watkinson, M. & Goldup, S. M. Macrocycle size matters: “small” functionalized rotaxanes in excellent yield using the CuAAC active template approach. Angew. Chem. Int. Ed. 50, 4151–4155 (2011).
Lewis, J. E. M., Modicom, F. & Goldup, S. M. Efficient multicomponent active template synthesis of catenanes. J. Am. Chem. Soc. 140, 4787–4791 (2018).
Cirulli, M. et al. Rotaxane-based transition metal complexes: effect of the mechanical bond on structure and electronic properties. J. Am. Chem. Soc. 141, 879–889 (2019).
Zhang, Z., Tizzard, G. J., Williams, J. A. G. & Goldup, S. M. Rotaxane PtII-complexes: mechanical bonding for chemically robust luminophores and stimuli responsive behaviour. Chem. Sci. 11, 1839–1847 (2020).
Hannam, J. S. et al. Controlled submolecular translational motion in synthesis: a mechanically interlocking auxiliary. Angew. Chem. Int. Ed. 43, 3260–3264 (2004).
Chao, S., Romuald, C., Fournel-Marotte, K., Clavel, C. & Coutrot, F. A strategy utilizing a recyclable macrocycle transporter for the efficient synthesis of a triazolium-based [2]rotaxane. Angew. Chem. Int. Ed. 53, 6914–6919 (2014).
Koenis, M. A. J. et al. Vibrational circular dichroism spectroscopy for probing the expression of chirality in mechanically planar chiral rotaxanes. Chem. Sci. 11, 8469–8475 (2020).
Coutrot, F., Waeles, P. & Gauthier, M. Challenges and opportunities in the post-synthetic modification of interlocked molecules. Angew. Chem. Int. Ed. 60, (2020).
Rowan, S. J. & Stoddart, J. F. Precision molecular grafting: exchanging surrogate stoppers in [2]rotaxanes. J. Am. Chem. Soc. 122, 164–165 (2000).
Gaedke, M. et al. Chiroptical inversion of a planar chiral redox-switchable rotaxane. Chem. Sci. 10, 10003–10009 (2019).
Corra, S. et al. Chemical on/off switching of mechanically planar chirality and chiral anion recognition in a [2]Rotaxane molecular shuttle. J. Am. Chem. Soc. 141, 9129–9133 (2019).
Martinez-Cuezva, A., Saura-Sanmartin, A., Alajarin, M. & Berna, J. Mechanically interlocked catalysts for asymmetric synthesis. ACS Catal. 10, 7719–7733 (2020).
Pairault, N. & Niemeyer, J. Chiral mechanically interlocked molecules—applications of rotaxanes, catenanes and molecular knots in stereoselective chemosensing and catalysis. Synlett 29, 689–698 (2018).
Mitra, R., Zhu, H., Grimme, S. & Niemeyer, J. Functional mechanically interlocked molecules: asymmetric organocatalysis with a catenated bifunctional bronsted acid. Angew. Chem. Int. Ed. 56, 11456–11459 (2017).
Pairault, N. et al. Heterobifunctional rotaxanes for asymmetric catalysis. Angew. Chem. Int. Ed. 59, 5102–5107 (2020).
Dommaschk, M., Echavarren, J., Leigh, D. A., Marcos, V. & Singleton, T. A. Dynamic control of chiral space through local symmetry breaking in a rotaxane organocatalyst. Angew. Chem. Int. Ed. 58, 14955–14958 (2019).
Cakmak, Y., Erbas-Cakmak, S. & Leigh, D. A. Asymmetric catalysis with a mechanically point-chiral rotaxane. J. Am. Chem. Soc. 138, 1749–1751 (2016).
Acknowledgements
S.M.G. thanks the European Research Council (Consolidator Grant Agreement number 724987), the EPSRC (EP/L016621/1) and the Leverhulme Trust (ORPG-2733) for funding and the Royal Society for a Wolfson Research Fellowship (RSWF\FT\180010). J.M.S. thanks the Royal Society for a Newton International Fellowship (NIF\R1\181686). A.W.H. thanks the University of Southampton for a Presidential Scholarship.
Author information
Authors and Affiliations
Contributions
M.A.J. synthesized rotaxane 4b. M.A.J. and A.d.J. developed the chiral interlocking auxiliary concept in collaboration with S.M.G. A.d.J. synthesized rotaxanes 8, 13–15, 23 and 28 with support from M.A.J. who provided synthetic intermediates. D.L. performed the stereochemical characterization of rotaxanes 4, 8, 13–15, 23 and 28. D.L. carried out the co-conformational analysis of rotaxane 4 and associated experiments. A.W.H. and J.M.S. developed the cross-coupling concept and collaborated to synthesize rotaxane 10. J.M.S. synthesized and characterized rotaxanes 18–20 and demonstrated the importance of the o-Me group using alkyne S87. A.W.H. synthesized and characterized rotaxane 12. G.J.T. collected the single-crystal X-ray diffraction data of rac-4b and solved and refined the structures. D.L. and A.W.H. managed the preparation of the Supplementary Information. S.M.G. directed the research. All authors contributed to the analysis of the results and the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental data, procedural details, synthesis and characterization data, NMR spectra, circular dichroism spectra, HPLC chromatograms, X-ray crystallographic data, discussions, Figs. 1–418 and Tables 1–6.
Supplementary Data 1
Crystallographic data for compound rac-4b(1). CCDC reference 2069804.
Supplementary Data 1
Crystallographic data for compound rac-4b(2). CCDC reference 2093365.
Rights and permissions
About this article
Cite this article
de Juan, A., Lozano, D., Heard, A.W. et al. A chiral interlocking auxiliary strategy for the synthesis of mechanically planar chiral rotaxanes. Nat. Chem. 14, 179–187 (2022). https://doi.org/10.1038/s41557-021-00825-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00825-9
This article is cited by
-
Controlling dynamics in extended molecular frameworks
Nature Reviews Chemistry (2022)
-
Mechanically axially chiral catenanes and noncanonical mechanically axially chiral rotaxanes
Nature Chemistry (2022)
-
Analysis of the host–guest complex formation involving bridged hexameric pyridinium–phenyl rings in the HexaCage6+ host in suit[3]ane: insights from dispersion-corrected DFT calculations for a nanometric mechanically interlocked device
Journal of Nanostructure in Chemistry (2022)