Lecanemab Sweeps Up Toxic Aβ Protofibrils, Catches Eyes of Trialists
Quick Links
With all the attention on aducanumab and donanemab these days (see previous stories in this series), lecanemab has been flying under the radar. Yet this often overlooked anti-amyloid antibody from Eisai/Biogen potently clears amyloid while producing less brain edema than its competitors. At the Clinical Trials on Alzheimer’s Disease conference, held in Boston and online November 9-12, Lars Lannfelt of Uppsala University, Sweden, made a case for lecanemab, aka BAN2401, being the strongest binder of aggregated Aβ among the current crop of anti-Aβ42 antibodies. Lannfelt led the BioArctic Neuroscience team that developed this antibody. Other talks at CTAD touted the consistency of lecanemab Phase 2 data, and added more evidence that it curbs tau pathology.
- Lecanemab binds protofibrils more tightly than fibrils, perhaps explaining lower ARIA rate.
- Use of plasma biomarkers brings down trial screening cost.
- Lecanemab has been selected for the first amyloid-tau concurrent therapy trial.
Lecanemab may soon become widely available; Eisai/Biogen have begun submitting data to the Food and Drug Administration to support its accelerated approval (Oct 2021 news). In the meantime, studies are ongoing, with this passive immunotherapy now in a trio of trials. CTAD speakers gave updates on screening improvements in the Phase 3 Clarity trial and the AHEAD 3-45 prevention study, which enrolls cognitively healthy people with amyloid plaques. Lecanemab has also been selected for the DIAN-TU Tau Next Generation trial, which enrolls people with an autosomal-dominant AD mutation. Tau NexGen will be the first trial to test combined amyloid and tau immunotherapies.
Randall Bateman of Washington University in St. Louis, who leads DIAN-TU, told Alzforum that combination therapy has long been the goal of the program. He believes the FDA’s approval of aducanumab opened the door for combination trials by creating demand for anti-amyloid agents. “The aducanumab approval changed the field’s perception of what is possible,” Bateman told Alzforum. “I think monotherapy trials’ days are numbered.”
Distinct Selectivity. Binding assays of three antibodies against different forms of Aβ indicate that lecanemab binds most strongly (dark gray) to protofibrils, while aducanumab and gantenerumab favor fibrils. [Courtesy of Linda Söderberg, BioArctic.]
A Protofibril Hog
Lecanemab differs from other antibodies in late-stage trials in that it was generated against synthetic Aβ protofibrils (Apr 2011 news). Protofibrils are large, soluble, β-sheet aggregates between 75 and 500 kD in size that are a major source of amyloid toxicity (Sehlin et al., 2012). By contrast, aducanumab was derived from the natural antibodies of healthy aged donors and recognizes aggregated Aβ, while gantenerumab was raised against fibrils. To pin down how these antibodies differ, Lannfelt tested them against each other in binding assays of Aβ42 monomers, oligomers, small protofibrils, large protofibrils, and fibrils. He used three different techniques: inhibition ELISA, immunodepletion, and surface plasmon resonance, where Aβ bound to a chip captures antibodies from solution. Lannfelt did not test donanemab, which recognizes a unique pyroglutamated form of Aβ truncated at the N-terminus.
Aducanumab was the weakest Aβ binder of the three. It did not recognize monomers or oligomers, and bound protofibrils transiently, with a fast on and off rate. It clung most strongly to fibrils. Gantenerumab also preferred fibrils over other forms, but did have some weak binding to monomers and oligomers, and glommed onto protofibrils as well. Overall, for both aducanumab and gantenerumab, binding strength increased with the Aβ aggregate size, but gantenerumab was the stronger Aβ binder no matter the species.

Protofibril Penchant. Inhibition ELISA shows that lecanemab binds small and large protofibrils with 25-100 times more affinity than does aducanumab, and 10 times more than gantenerumab. [Courtesy of Lars Lannfelt.]
Lecanemab had a different profile. It did not bind monomers or small oligomers, but did recognize larger oligomers in the 6- to 12-mer range, and it bound fibrils as well. However, it bound with the highest affinity to the mid-sized species, the protofibrils (see image above for three-way comparison). In head-to-head comparisons, lecanemab bound small protofibrils of 75-300 kD 10 times as strongly as did gantenerumab, and 100 times more than aducanumab. For larger protofibrils of 300-500 kDa, lecanemab bound 25 times as strongly as aducanumab.
If lecanemab mostly targets protofibrils, how does it clear fibrillar plaque? Lannfelt believes the antibody shifts the equilibrium in the brain by depleting soluble species. This would cause more Aβ to solubilize off plaques, dissolving these deposits, he suggested. Because Aβ can equilibrate between forms, targeting one species often affects others. For example, a prior study found that aducanumab suppresses oligomer formation by coating fibrils and preventing secondary nucleation of those smaller species on their surface (Sep 2020 news).
The different binding properties of each antibody may influence their clinical effects, Lannfelt noted. For example, ARIA, a type of brain edema caused by immunotherapy, may arise through antibodies binding to fibrils deposited along blood vessel walls, causing inflammation and leakiness of these vessels, he suggested. If so, lecanemab’s lower affinity for fibrils may explain its lower rate of ARIA compared to other antibodies, with an incidence of about 10 percent rather than 35.
Phase 2 Data Revisited
Why has lecanemab not gotten more attention? Perhaps it has to do with lingering concerns over the Phase 2 trial data. That study used an adaptive Bayesian design, which allows researchers to adjust parameters on the fly to favor the most effective dose, speeding up the trial (Nov 2012 conference news). The trial first read out at 12 months, but at that time failed to meet its prespecified endpoint of dramatically slowed decline on the ADCOMS composite (Dec 2017 news). By 18 months, however, lecanemab had slowed cognitive and functional decline by 30 percent on the primary outcome measure, similar to the performance of aducanumab and donanemab (Jul 2018 news; Jul 2018 conference news; Nov 2018 conference news).
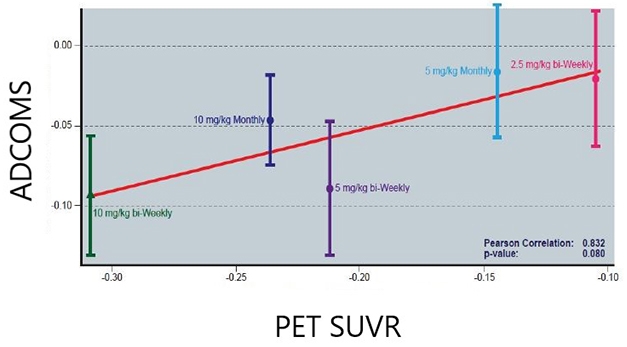
Less Amyloid, Less Decline? In the lecanemab Phase 2 trial, the more plaque removed, the less decline in the ADCOMS. Data shows adjusted mean differences from the placebo group. [Image Courtesy of Eisai.]
At CTAD, Donald Berry of the University of Texas MD Anderson Cancer Center in Houston put these findings into context. Berry’s consulting group designs Bayesian trials, including the lecanemab trial. He noted that the goal of the 12-month endpoint was to show “super-superiority” over placebo, defined as an 80 percent probability that lecanemab would slow decline by 25 percent or more. At 12 months, lecanemab fell short of this, with a 64 percent probability of being super-superior to placebo. However, at this early point in time lecanemab was 98 percent likely to be superior to placebo, indicating there was a genuine drug effect.
Berry next addressed the robustness of the findings, analyzing the three cognitive endpoints—ADCOMS, ADAS-Cog14, and CDR-SB—using six different statistical methods. No matter the method or the end point, the findings were consistent, he found. In every analysis, the two highest doses of lecanemab, 10 mg/kg monthly and 10 mg/kg biweekly, were superior to placebo in a dose-dependent fashion. The ADAS-Cog14 was the most sensitive measure, with p values obtained by the six methods typically significant at the 0.01 level; CDR-SB was the least sensitive, with three of the six methods significant at the 0.05 level, and the others missing the mark. Overall, the consistency of the findings suggests a robust effect, Berry argued. He noted that regulators accept Bayesian designs for registration trials, and they have been used successfully in other fields, such as for Eli Lilly’s diabetes drug Trulicity.
Plasma Phospho-Tau Strengthens the Case
Eisai’s Chad Swanson added data from the open-label extension of this Phase 2 trial. Participants had been off lecanemab for an average of two years when the OLE started. As previously reported, the cognitive benefit seen on the highest doses was maintained over the time gap, and the banished plaques largely stayed gone (Dec 2019 conference news; Aug 2021 conference news). At CTAD, Swanson reported a strong association between plaque clearance in the OLE and slower decline on ADCOMS, with an eye-popping correlation coefficient of 0.83 at the treatment-group level. This contrasts with aducanumab Phase 3 data, where critics have pointed out a weak relationship between plaque clearance and cognitive benefit.
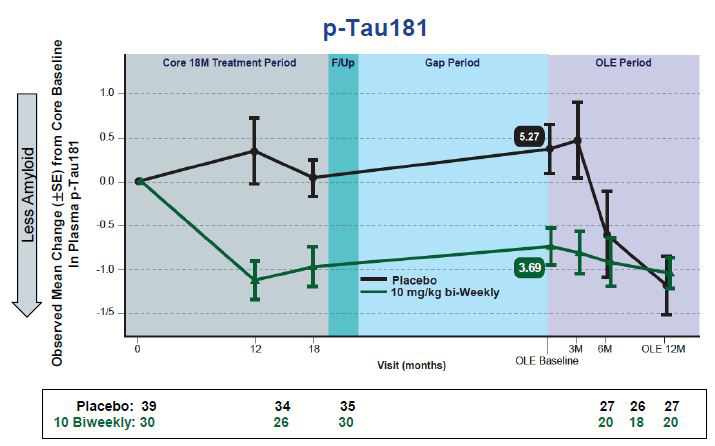
Plasma p-Tau Falls. Changes in plasma p-tau181 in people taking lecanemab mirror those previously reported for plaque clearance. [images courtesy of Eisai.]
New at CTAD were plasma p-tau181 data from this OLE. The recent availability of sensitive plasma biomarker tests is enabling researchers to mine new data from past trials, for example the analysis of plasma p-tau181 from the aducanumab Phase 3 trials (Nov 2021 conference news). Plasma biomarkers are also improving screening, such as the use of plasma p-tau in the Phase 3 donanemab trial (Nov 2021 conference news). For this OLE, Swanson reported that changes in plasma p-tau181 tracked with changes in amyloid PET and plasma Aβ42/Aβ40 at the level of individual participants. In the Phase 3 study, researchers will test whether plasma p-tau also correlates with the slowing of cognitive decline, Swanson said.
The relationship between plasma p-tau and brain amyloid suggests that lowering plaques slows tau phosphorylation, Bateman noted. “That’s encouraging from a biological standpoint,” he said, but added that these plasma biomarkers need to be validated with clinical and cognitive measures.
Christopher van Dyck of Yale University in New Haven, Connecticut, agreed that plasma biomarkers need further validation before they can be used to predict clinical outcomes. Nonetheless he was impressed with the lecanemab data, noting that the cognitive benefit maintained off drug strongly suggests a disease-modifying effect. “There’s a lot to like in these data,” van Dyck said.
Streamlining Screening to Bring Down Cost
Lecanemab’s star may be on the rise, with the antibody now in three large trials. Swanson updated the CTAD audience on the Phase 3 Clarity trial, which now has 1,795 participants. Enrollment is complete in all 14 countries, except China. Demographics are similar to the Phase 2 trial, except the participants are more diverse. Phase 2 was 90 percent Caucasian, whereas Clarity’s population is 17 percent Asian, 13 percent Hispanic, and 3 percent black (Nov 2020 conference news).
Eisai’s Michelle Gee covered new screening procedures in Clarity. Screen failures are particularly costly for Alzheimer’s trials now because of the expense of PET scans. This can be minimized by using a tiered system that eliminates many applicants before imaging, Gee said. For Clarity, researchers developed a five-step process. In the first visit, participants provide a medical history and demographic data, and take three cognitive tests. These are the MMSE and the Wechsler Memory Scale Logical Memory tests I and II. About 35 percent of people who did not qualify for Clarity were eliminated at this first step, most due to their performance on the LM-II, which assesses delayed recall.
Step 2 comprises a physical exam, including ECG and labs, while step 3 adds an MRI scan. Relatively fewer screen fails happened at these stages—16 and 12 percent of the total, respectively. Step 4 is more comprehensive: Participants took numerous cognitive and functional tests and repeated the physical exam, MMSE, and Wechsler tests to obtain baseline data. Nine percent of screen fails happened at this stage. Only at step 5 did participants undergo amyloid and tau PET and/or lumbar puncture. Despite the extensive prescreening, 29 percent of screen fails happened here, mostly due to negative amyloid tests. Overall, 5,972 people needed to be screened to obtain 1,795 participants, a 70 percent overall fail rate. Still, Gee said the tiered system lowered cost and recruitment time as well as the burden on potential participants and study sites, since the majority of screen failures happened early in the process.
Likewise, Reisa Sperling of Brigham and Women’s Hospital, Boston, is refining screening methods for the AHEAD 3-45 secondary prevention trial of lecanemab. She found that prescreening using the plasma Aβ42/Aβ40 ratio helped select for amyloid-positive participants and dramatically cut down the number of PET scans needed (Nov 2021 news).
First Amyloid-Tau Combination Trial
The third trial testing lecanemab has a different design. DIAN-TU researchers had already planned Tau NexGen to be a tau-based study testing Eisai’s anti-tau antibody E2814 as the first of three trial arms when aducanumab received FDA approval last summer (Mar 2021 news; Jun 2021 news). Researchers surveyed DIAN participants, who carry inherited AD mutations that accelerate plaque formation, to gauge their interest in taking an anti-amyloid therapy (Aug 2021 news). The results were unequivocal: patients and families demanded access to anti-amyloid treatment, Bateman told Alzforum.
Researchers saw advantages in concurrent therapy, too. “We think tau therapy is more likely to succeed if we first remove amyloid plaques, which are driving tauopathy,” Bateman explained. Targeting tau in the continued presence of amyloid could be an uphill battle, Bateman said.
Now, participants will take lecanemab as well as E2814, but on a schedule that depends on their disease stage. Presymptomatic participants, who have less plaque, will take only E2814 or placebo for one year, as originally planned, then add lecanemab. Symptomatic participants will take lecanemab alone for six months to clear plaque, then add E2814 or placebo. This design will allow researchers to compare the effect of E2814 or lecanemab alone to the effect of both together. The trial will run for two years, with the primary endpoint being change in tau PET, and a secondary endpoint of CSF p-tau217. If these biomarkers are positive, the trial will run for two more years to assess cognitive change.
What about the other two arms of the Tau NexGen trial? Researchers have not yet chosen the therapies, but have decided they will hit different mechanisms, such as an anti-aggregation or tau anti-sense agent (Aug 2021 news). Whatever the tau drug, participants in all arms will take lecanemab. Bateman noted that one reason for selecting this antibody over others was because Eisai agreed to make it available for all subsequent trial arms, even those that evaluate non-Eisai tau agents. This allows researchers to use a single pooled control group, increasing the trial’s power, since everyone will be on the same anti-amyloid therapy for comparison. “All four [anti-Aβ] antibodies that clearly demonstrated evidence of amyloid removal were on the table. All had pros and cons,” Bateman noted. “It was a tough decision.”—Madolyn Bowman Rogers
References
Therapeutics Citations
News Citations
- Lecanemab Follows Aduhelm’s Path to Accelerated Approval
- Barcelona: Antibody to Sweep Up Aβ Protofibrils in Human Brain
- Of Four Aβ Antibodies, Only Aducanumab Stems Tide of Toxic Oligomers
- CTAD: Adaptive Antibody Trial to Try Bayesian Statistics
- No Man’s Land: Neither Early Success nor Failure for BAN2401
- Topline Results: 18 Months of BAN2401 Might Work
- BAN2401 Removes Brain Amyloid, Possibly Slows Cognitive Decline
- Second Look at BAN2401 Data Still Positive, Despite Snafu
- Amyloid Clearance: Check. Cognitive Benefit: Um … Maybe.
- Lecanemab Post Hoc: Is Continual Treatment Required for Cognitive Benefit?
- Aduhelm Lowers Tau; Registry to Track Real-World Performance
- Donanemab Phase 3 Puts Plasma p-Tau, Remote Assessments to the Test
- BANish Aβ? BAN2401 Antibody Makes Its Move in Phase 3 Program
- Plasma Aβ—First Sign of AD, But Tough to Measure Prospectively?
- Aiming at the Tangle’s Heart? DIAN-TU Trial to Torpedo Tau’s Core
- Aducanumab Approved to Treat Alzheimer’s Disease
- Aduhelm Approval Reverberates Through Research
- Antisense Therapy Stifles CSF Tau in Mild Alzheimer’s Disease
Paper Citations
- Sehlin D, Englund H, Simu B, Karlsson M, Ingelsson M, Nikolajeff F, Lannfelt L, Pettersson FE. Large aggregates are the major soluble Aβ species in AD brain fractionated with density gradient ultracentrifugation. PLoS One. 2012;7(2):e32014. PubMed.
Other Citations
Further Reading
Annotate
To make an annotation you must Login or Register.

Comments
No Available Comments
Make a Comment
To make a comment you must login or register.