Roundtable Discussion: Mikhael Breaks Down Options for Long-term Treatment of Patients With Newly Diagnosed Multiple Myeloma
A 51-year-old man presented with worsening fatigue on exertion and pallor with an ECOG performance score of 1. He eventually received a diagnosis of stage II standard-risk multiple myeloma after testing and examination.
Joseph Mikhael, MD

During a Targeted Oncology Case-Based Roundtable event, Joseph Mikhael, MD, professor, Applied Cancer Research and Drug Discovery Division, Translational Genomics Research Institute, an Affiliate of City of Hope, discussed the case of a 51-year-old patients with newly-diagnosed multiple myeloma.
MOSS: As far as prognosis goes, of course this has changed quite a bit in the past 15 to 20 years, and I try to be very upbeat with patients. Certainly, they do not have particularly high [expectations], so I try to be upbeat about that with patients that can now survive for many years with multiple myeloma. However, I do emphasize, even at the beginning, [that] at this time, it is not a curable disease and that virtually everyone will progress at some time during the future.
So we don’t want to mislead them that...whatever we use for first-line therapy is going to cure them. I want them prepared for a long-term treatment. I do not like to beat them over the head with that, but I want that to be up front. As far as transplant goes...for me, this has been a big problem.
MIKHAEL: I think you made some good points about [multiple myeloma] not being curative. The median survival amount is really between 8 and 10 years for people that do not have high-risk disease.1
CHARU: Usually I refer my patients for transplant just not by their age, especially in younger patients. I also discuss with them that this is not a curable disease, but based on the long-term data that we have, at least the 5-year survival is seen in more than 55% of patients. And [30% to 37%] of the patients even go on for 10 years, even though it is not curable disease; it is just treatable disease.
MIKHAEL: The prognosis of myeloma does continue to improve, not only because we are bringing great therapies at the end in heavily relapsed disease...where therapies get their first approval, but [also because we’re] bringing out treatments earlier on. We are now becoming more and more intense in our treatments early on, as I was saying.
YEH: Do you strive for MRD [minimal residual disease] negativity before transplant?
MIKHAEL: Looking at those that had MRD negativity going into transplant and finding out that those people did better in the long run, of course they are going to do better. Responders do better. We have always known that in myeloma.
I think we all recognize that we want some chemotherapy sensitivity. We all want to see the numbers going down. Again, there is a difference in opinions about this in the myeloma community. Some centers are very vigilant and are like, “Unless someone achieves a VGPR [very good partial response] or unless someone has less than 10% plasma cells, I am not going to take them to transplant.”
I think that [we have to look at patients with multiple myeloma as more complicated to treat]. It is not a cookie-cutter disease. There are people who get deep responses and, sadly, relapse quickly. We have long-term survivors with myeloma who have never achieved a complete remission, so to try to put everybody into the same bucket or approach is, I think, dangerous.
The general approach is you want to see a good depth of response. At least a partial response [PR] and, perhaps, ideally, a VGPR, but not necessarily so [in every case]. If the patient has standard-risk disease, especially if the patient has hypodiploidy or extra copies of chromosomes, those people tend to have a long-term response and often go back to a monoclonal gammopathy of undetermined significance [MGUS]–like state.
So it is hard for them to get rid of all their monoclonal proteins. If we do say that we do not think that the patient is having an adequate response to induction, it is reasonable to try a second approach, but not a third, fourth, or fifth. If you are going to transplant, give the transplant [its] opportunity because we know that transplant will upgrade responses. We also have some forms of myeloma that are just not very sensitive to our novel treatments but remain quite sensitive to alkylators.
DY: I refer everybody to transplant, and let the experts decide whether they are eligible or not. Except, of course, if they have really poor performance.
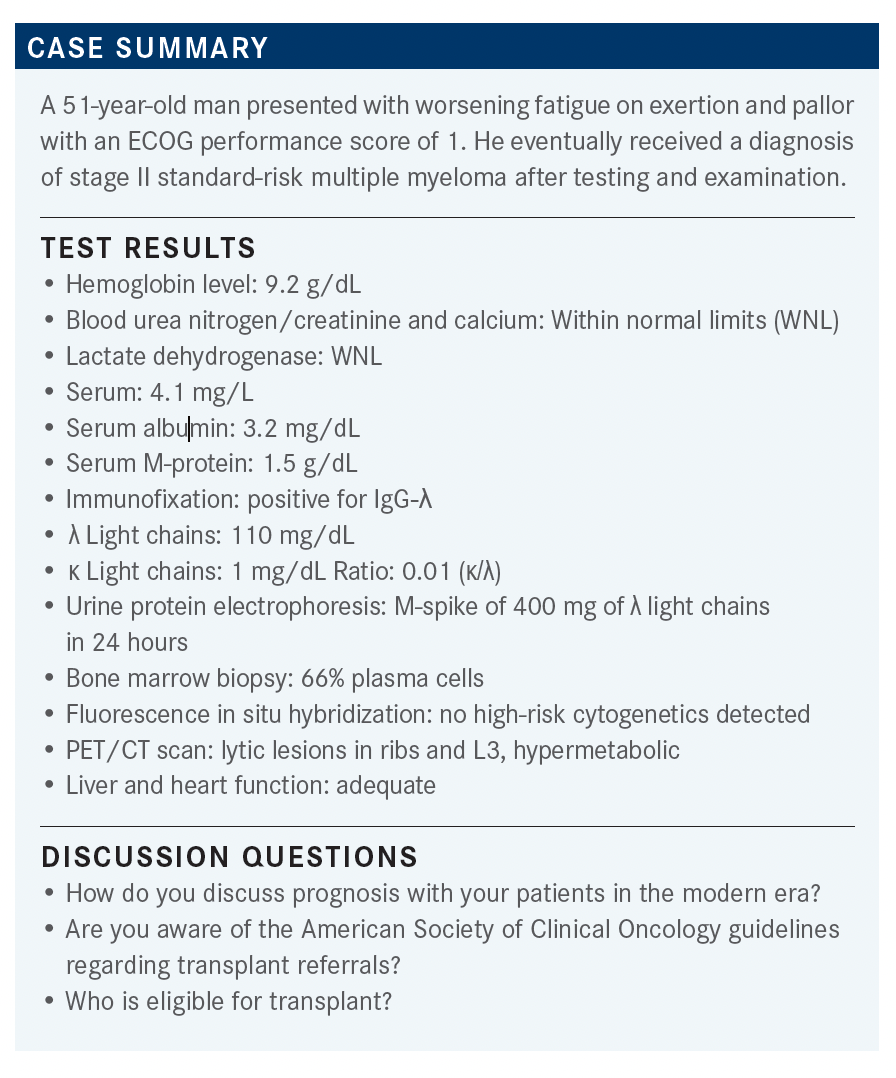
MIKHAEL: When we look at standard regimens, we would typically go to the NCCN [National Comprehensive Cancer Network] guidelines, which are less guideline-ish and are more laundry-list style. They are there to remind us of what is available, and currently bortezomib [Velcade], lenalidomide [Revlimid], [and] dexamethasone, or VRd, tends to be the most preferred regimen.2
Sometimes we use the CyBorD regimen, which is cyclophosphamide, bortezomib, and dexamethasone. But there may be patients eligible for carfilzomib [Kyprolis], lenalidomide, or dexamethasone, and even daratumumab [Darzalex] and lenalidomide plus dexamethasone, and occasionally even ixazomib [Ninlaro], lenalidomide, plus dexamethasone.
CHARU: I think the quadruplet regimen looks better overall, and I think I refer all my patients for transplant evaluation to begin with. I think for transplant-eligible patients, it is probably beneficial to get a transplant.
MIKHAEL: I think the bigger question that may threaten transplant with time is potentially...CAR [chimeric antigen receptor] T-cell therapy. That is a whole separate discussion because we are seeing such remarkable responses there.
YEH: Is MRD a surrogate for survival?
MIKHAEL: I would say that in general, MRD negativity is a surrogate marker for both progression-free and overall survival, with the caveat that there is about 10% to 15% of patients with multiple myeloma who never really get a deep response but live a long time. So that can color the mix a little bit, and there are those patients who go back to almost a MGUS-like state, and those are never high-risk patients.
MOSS: I was disappointed with the results. I was disappointed that it did not show the carfilzomib was not a significant improvement over bortezomib, so VRd still holds its place.
MIKHAEL: Yes, and there are different ways of interpreting that. A lot of people...get very passionate about this. My short version would be [to] remember the ENDURANCE study [NCT01863550] that looked at the very people who were probably not using carfilzomib3—patients not going to transplant and patients without high-risk cytogenetics. I think that is probably where it has a greater role because I do think that it is a more potent proteasome inhibitor. I think most people would agree with that, but that is why now we are exploring more KRd [carfilzomib, lenalidomide, and dexamethasone] studies in that transplant-eligible space.
SHETH: Certainly, the data for the 4-drug combination [are] compelling, but you...just can’t ignore [the toxicity] on the GRIFFIN study [NCT02874742].⁴ The age cutoff was for patients who were [aged 70 years], whereas in the European study, they allowed patients [older] than [65 years]. I think that is something to really investigate very closely when determining whether 4 drugs is applicable to our real-world patient population.
In the end, it is really going to depend on what we are achieving by giving the 4 drugs. If we can then say, “Look, if you get that stringent complete response, it is now showing us that you do not necessarily need a transplant,” [then] I think there would be a greater role for advocating for that use. It is nice for us to use these drugs in a sequential manner so that we do not run out of options when [patients] progress as they get older and as they live with more years with [the disease]. I think we just still need more long-term data with that quadruplet therapy.
MIKHAEL: I think that the burden of proof must be on it because there is a lot at stake, as you said. There is the clinical toxicity. There is the financial toxicity. There are the long-term data not being clear. I thought it was interesting...that in the design of GRIFFIN they gave the daratumumab only for 2 years of maintenance because most people will get beyond those 2 years. A lot of people when they relapse, they are not relapsing on daratumumab. So the phase 3 version of GRIFFIN is taking a different approach. It is using MRD negativity to decide on the continuance or noncontinuance with treatment.
I do think that we need a little bit more time to make that decision, but I think there is a strong move to the quadruplets. There [was a] significantly greater fraction of patients getting a deeper response, and ironically, the transplant contributions seemed to be less than the induction and the consolidation bit. So I think you make a very good point that [maybe] with time, [but] I would not say necessarily we are ready to dispense transplant.
REFERENCES:
1. Key statistics about multiple myeloma. American Cancer Society. Updated January 12, 2021. Accessed October 28, 2021. https://bit.ly/3CAaLzT
2. NCCN. Clinical Practice Guidelines in Oncology. Multiple myeloma, version 3.2021. Accessed October 28, 2021. https://bit.ly/2ZJBzjs
3. Kumar SK, Jacobus SJ, Cohen AD, et al. Carfilzomib or bortezomib in combination with lenalidomide and dexamethasone for patients with newly diagnosed multiple myeloma without intention for immediate autologous stem-cell transplantation (ENDURANCE): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21(10):1317-1330. doi:10.1016/ S1470-2045(20)30452-6
4. Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936-945. doi:10.1182/ blood.2020005288

Advances in Subsequent Therapies Shake Up Sequencing of ccRCC Treatment
April 25th 2024With the approval of belzutifan and other newer data for treating patients with recurrent renal cell carcinoma, the state of subsequent therapies is advancing beyond the reuse of frontline options with impacts on duration of response and quality of life.
Read More
Age, Disease Burden Are Factors in Early Use of Selinexor in Multiple Myeloma
April 22nd 2024During a Case-Based Roundtable® event, Jonathan L. Kaufman, MD, discussed treatment approaches and the tolerability of a selinexor-containing regimen in a patient with relapsed/refractory multiple myeloma in the first article of a 2-part series.
Read More
Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More