Abstract
Limited data are available on antimicrobials exposure and microbiology evolution in pediatric acute myeloid leukemia (AML) patients underwent antimicrobials prophylaxis. To assess the effectiveness of antimicrobials prophylaxis, antibiotic susceptibilities of bacteria, and exposure of antimicrobials during intensive chemotherapy for AML patients, 90 consecutive de novo AML patients aged 0–18 years between January 1, 1997 and March 31, 2018 were enrolled. Vancomycin, ciprofloxacin and voriconazole prophylaxis was administered from January 1, 2010. During the preprophylaxis period, January 1997 to December 2009, 62 patients experienced a total of 87 episodes of bloodstream infection (BSI) and 17 episodes of invasive fungal infection (IFI) among 502 courses of chemotherapy. In contrast, 16 episodes of BSI occurred and no IFIs were reported to occur in 28 patients who received 247 courses of chemotherapy in the prophylaxis period. Patients who received antimicrobial prophylaxis had a significant reduction of BSI, IFI, and febrile neutropenia in comparison with patients without prophylaxis. Exposure to amikacin, carbapenem, amphotericin B was reduced in the prophylaxis period. Imipenem susceptibility of Enterobacter cloacae as well as vancomycin susceptibility of Enterococcus species were reduced in the prophylaxis period. At the time of the last follow up, patients with prophylaxis had a better subsequent 5-year overall survival rate than those without prophylaxis. Prophylactic antimicrobials administration in children with AML who undergo chemotherapy can significantly reduce the rates of life-threatening infection, exposure to antimicrobials, and might result in a better outcome.
Similar content being viewed by others
Introduction
Outcomes for pediatric acute myeloid leukemia (AML) patients have improved remarkably with a 70% improvement rate over the past decades1. Risk-directed treatment strategy, adjusted chemotherapy dosing or timing and intensification of therapy for disease eradication, better supportive-care measures to reduce early death and treatment-related mortality, and efficacy of salvage therapy contributed to this improvement2,3,4,5. Approximately 60% of children with AML who underwent chemotherapy experienced life-threatening infections with a reported cumulative infection-related mortality (IRM) of 6 to 11%6,7. To reduce the IRM, an approach of prophylaxis with antibiotics and antifungal agents in pediatric AML patients was reasonable and has been reported5,8,9,10. The use of prophylactic antibiotics in adult AML patients with afebrile neutropenia following chemotherapy is supported by meta-analyses of randomized trials demonstrating reduced the occurrence of clinically documented infections and risk of infection-related death11. Data describing the effectiveness of the use of antimicrobial prophylaxis in pediatric AML patients with afebrile neutropenia are limited5,8,9,10. Furthermore, there has been no detailed report on the comparison of antimicrobial exposure and changes in bacterial susceptibility during antimicrobial prophylaxis in pediatric AML patients. The primary objective of the current study was to investigate the effectiveness of prophylaxis with antibiotic and antifungal agents in the prevention of bloodstream infection (BSI), invasive fungal infection (IFI), febrile neutropenia (FN), and outcome during intensive chemotherapy for AML patients. A secondary aim was to assess antimicrobials susceptibilities of the major gram-negative bacteria (GNB), gram-positive bacteria (GPB), and Candida species, and the exposure of antimicrobials during the study period.
Materials and methods
Patients and protocols
This was a single-center, observational cohort study of patients with newly diagnosed AML at Mackay Children’s Hospital in Taipei, Taiwan. From January 1, 1997 through March 31, 2018, 90 consecutive children with AML who were younger than 18 years old and who did not have Down syndrome, acute promyelocytic leukemia, or therapy-related AML were enrolled in this study. The Institutional Review Board of MacKay Memorial Hospital approved the study and all of the participants or their guardians provided written, informed consent of AML treatment, in accordance with the Declaration of Helsinki. Patients with newly diagnosed AML were treated with the Taiwan Pediatric Oncology Group (TPOG)-AML-97A protocol (activated in January 1997) consisting of induction chemotherapy, post-remission high-dose (HD) and modest-dose (MD) chemotherapy as previously described (see Supplemental Figure S1 online)12,13. Minimal residual disease (MRD) measurement for AML with RUNX1-RUNX1T1, CBFB-MYH11, MLLT3-KMT2A fusion was started in 2013.
From January 1, 2010 to March 31, 2018, prophylaxis with antibiotic and antifungal agents was administered to afebrile neutropenic patients with AML who received induction as well as post-remission HD and MD chemotherapy. Each chemotherapy course was analyzed separately for occurrence of BSI, probable and proven IFIs, FN during the preprophylaxis period (1997–2009) and prophylaxis period (January 1, 2010–March 31, 2018). Microbiological organisms of BSI and IFI, treatment outcome, antibiotic susceptibilities of the major GNB and GPB at the study institution and exposure of antimicrobials for any causes during AML-97A chemotherapy were recorded to compare the preprophylaxis and the prophylaxis periods. The outcomes of the AML were censored on September 30, 2020, with a duration after completion of chemotherapy of more than 2 years and 6 months.
Antimicrobial prophylaxis protocols
Oral ciprofloxacin (at a dose of 300 mg/m2 every 12 h) and voriconazole (7 mg/kg every 12 h, maximum dose of 200 mg) were administered once an afebrile patient’s absolute neutrophil count (ANC) ≤ 0.5 × 109/L was reached and expected to last ≥ 7 days during chemotherapy. The incidence of GPB in children with AML treated at study institution from January 1997 to December 2012 was 5%, of which two patients resulted in death, so we started to initiated Vancomycin (400 mg/m2 administered intravenously every 12 h) in patients at the onset of neutropenia since January, 2013. Therapeutic drug monitoring (TDM) of voriconazole was performed to ensure efficacy and limit toxicity in patients receiving 7 days of voriconazole prophylaxis (1–5 mcg/mL as targeted trough level)14. TDM of vancomycin was not routinely monitored while its toxicities and tolerance were recorded. Prophylaxis with antibiotic and antifungal agents was discontinued once the ANC recovered to > 0.1 × 109/L post nadir. All patients were placed on Pneumocystis jirovecii prophylaxis with trimethoprim–sulfamethoxazole.
Management of febrile neutropenia
At the study institution, management strategies for a patient with febrile neutropenia included 2 blood cultures for bacteria obtained via the central venous catheter (if present) and a peripheral vein. Cefuroxime and amikacin were used as empirical antibiotics during two time periods of study. Prophylactic vancomycin and ciprofloxacin were withdrawn while voriconazole prophylaxis continued once patients experienced febrile neutropenia. These guidelines did not change during the study period.
Definitions of infectious episodes
The National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 5.0)15 was applied to define all infection events in which ≥ grade 3 were recorded. FN was defined as a single core body temperature ≥ 38.3 °C or ≥ 38.0 °C persisting at least one hour in context of neutropenia. BSI was defined as one recognized pathogen that was isolated from ≥ 1 blood culture without relation to an infection at another site, and clinical signs of systemic infection16. If one common commensal organism (e.g., methicillin-resistant coagulase-negative staphylococci) was isolated from 2 blood cultures among those patients with ANC ≤ 0.5 × 109/L, it was recorded as a true pathogen of BSI. All BSI in children at any time who underwent AML-97A chemotherapy was reported. IFIs, including yeasts and molds, were graded as proven, probable, or possible in accordance with the European Organization for the Research and Treatment of Cancer/Mycoses Study Group criteria17. Only proven and probable IFI were recorded for analysis in the current study.
Assessment of the exposure to antimicrobials
Specific antimicrobial days were calculated for each patient as the simple proportion of cumulative chemotherapy days on which a specific antimicrobial was administered (excluding Pneumocystis jirovecii pneumonia prophylaxis). Each antimicrobial exposure was calculated and compared between the preprophylaxis and prophylaxis periods. Antibiotics were grouped as follows: cephalothin or cefuroxime, ceftazidime, amikacin, piperacillin/tazobactam, carbapenem, and teicoplanin. Antifungal agents were grouped as follows: conventional or liposomal amphotericin B, caspofungin, and triazoles including fluconazole, itraconazole, and posaconazole.
Assessment of antimicrobials susceptibility
At the study institution, bacterial antibiotic susceptibility monitoring and recording have started since 2002 and antifungal susceptibility of Candida species has been available since 2017. The antibiotics susceptibility for the most common 6 GNB including Escherichia coli (E. coli), Klebsiella pneumoniae (K. pneumoniae), Enterobacter cloacae (E. cloacae), Pseudomonas aeruginosa (P. aeruginosa), Citrobacter freundii (C. freundii), Acinetobacter baumannii (A. baumannii) and 3 GPB including Staphylococcus aureus (S. aureus), Enterococcus species (Enterococcus spp.), Coagulase-negative Staphylococci (CoNS) in pediatric AML patients from 2002 to 2019 were recorded. The results of antibiotic susceptibility for every bacterium were compared individually between the preprophylaxis and prophylaxis periods. We focused on the change in antibiotic susceptibility of the bacteria during the prophylaxis period.
Statistical analysis
Comparisons of developing BSIs, IFIs, and FN presented as the proportion of chemotherapy courses, clinical and genetic features, and mortality from infections between the 2 periods were estimated using the chisquare test. Hierarchical linear modeling (HLM) was used to adjust covariates including age, duration of neutropenia, oral mucositis, and parenteral nutrition therapy for potential confounding influences of the occurrence of BSIs, IFIs, and FN during preprophylaxis and prophylaxis periods. The Kruskal–Wallis test was used to compare course and cumulative days of chemotherapy. The Mann–Whitney U test was used to compare the antimicrobials exposure and antibiotic susceptibility between the 2 periods. Kaplan–Meier estimates were used to plot overall survival (OS), event-free survival (EFS), and to graph the time to first event for FN, BSI, or IFI between the preprophylaxis and prophylaxis periods and were compared using the log-rank test. A P value < 0.05 was considered to be statistically significant. SPSS statistical software (version 21; SPSS Inc, Chicago, Ill) was used to perform the statistical analyses.
Results
Presenting features of the 90 children were shown in Table 1. Patient’s flow chart during the study was shown in Supplemental Figure S2.
Effectiveness of antimicrobials prophyalxis
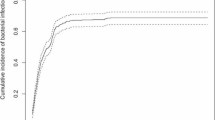
There were 87 episodes of BSI and 17 episodes of IFI reported during the preprophylaxis period (see Supplemental Table S3 online). In the prophylaxis period, 16 episodes of BSI and no IFI occurred during chemotherapy. K. pneumoniae (28 isolates) was the leading microorganism of BSI and Aspergillus spp. (11 isolates) of IFI in the preprophylaxis period, whereas E. coli was isolated most commonly (7 isolates) in the prophylaxis period. All episodes of IFI occurred during periods of neutropenia while 3 episodes of BSI occurred without neutropenia during the preprophylaxis period and one during the prophylaxis period. In the prophylaxis period, the reduction in the rates of BSI in patients with AML who were receiving induction or HD chemotherapy and IFI in those receiving HD chemotherapy reached a statistically significant level (Table 2). Patients receiving antimicrobial prophylaxis during HD chemotherapy had a lower cumulative incidence of BSI and IFI in Kaplan–Meier analysis (Fig. 1). The reduction of BSI during the prophylaxis period was also remarkable as using HLM analysis after adjustment for covariates (see Supplementary Table S4 online).
The frequencies of FN in induction, HD, or MD chemotherapy were reduced significantly during the prophylaxis period (Table 2), and HLM analysis (Supplementary Table S4) showed similar results. However, a lower cumulative incidence of FN was only in induction or MD chemotherapy during the prophylaxis period under Kaplan–Meier analysis (Fig. 1). Thirteen patients with AML died of infection during the preprophylaxis period (7 of BSIs and 6 of IFIs). In contrast, only one patient with AML, M7 subtype died of P. aeruginosa BSI with typhilitis after his fourth course of HD chemotherapy during the prophylaxis period.
At the time of last follow-up (September 30, 2020), the 5-year EFS rate was 51.6% [39.3% to 63.9%] and the 5-year OS rate was 54.8% [42.5 to 67.1%] for 62 patients during the preprophylaxis period; for 28 patients during the prophylaxis period, the rate were 70.6 [53.4% to 87.8%] and 78.6% [63.3% to 93.9%] (Fig. 2).
Effect of prophylaxis on antimicrobials exposure and susceptibility
Ciprofloxacin, vancomycin, and voriconazole exposure was significantly greater during prophylaxis period than preprophylaxis period. Antimicrobial exposure was presenting as specific antimicrobial days. There was a concomitant reduction in exposure to carbapenem, amikacin, conventional/liposomal amphotericin B, or caspofungin (P < 0.001 for all comparisons; Fig. 3).
Data of specific antimicrobial days are shown in a box plot; each box plot illustrates the upper and lower quartile (box), median (line inside box), adjacent values (whiskers), and outliers (open circles). Patients receiving antimicrobial prophylaxis had less exposure to carbapenem, amikacin, amphotericin B, or caspofungin when compared with those receiving no prophylaxis (P < 0.001, P < 0.001, P < 0.001, and P < 0.001, respectively).
The antibiotic susceptibility of the most common six GNB and three GPB at the study institution from 2002 to 2019 is shown in Fig. 4. The cefuroxime susceptibility of E. coli and K. pneumonia as well as imipenem susceptibility of E. cloacae and A. baumannii were reduced significantly during the prophylaxis period. Moreover, there was a concomitant rising of amikacin susceptibility of E. coli, K. pneumoniae, E. cloacae, P. aeruginosa, C. freundii during the prophylaxis period when compared with the preprophylaxis period. In GPB, the ampicillin/sulbactam, linezolid, teicoplanin, and vancomycin susceptibility of Enterococcus spp. had a significant reduction during the prophylaxis period.
The antibiotic susceptibility of 6 GNB (a) and 3 GPB (b) between the preprophylaxis and prophylaxis period are shown. Data of specific antimicrobial days are shown in a box plot; each box plot illustrates the upper and lower quartile (box), median (line inside box), and adjacent values (whiskers). (a) During the prophylaxis period, cefuroxime (CXM) susceptibility of E. coli or K. pneumonia were reduced significantly (P = 0.027, P = 0.01, respectively); imipenem (IPM) susceptibility of E. cloacae or A. baumannii were also reduced (P = 0.009, and P = 0.002, respectively); a concomitant rising of amikacin (AN) susceptibility of E. coli, K. pneumoniae, E. cloacae, P. aeruginosa, C. freundii was found (P = 0.042, P = 0.001, P = 0.007, P = 0.003, and P = 0.001, respectively). (b) The ampicillin/sulbactam (SAM), linezolid (LZD), teicoplanin (TEC), or vancomycin (VA) susceptibility of Enterococcus species had a significant reduction during the prophylaxis period. The antifungal susceptibility of Candida species is shown in (c) from 2017 to 2020. *, values are presenting as the median number of infection episodes from any site of body per year from 2002 to 2019 for bacteria and from 2017 to 2020 for Candida species. AMB, amphotericin B; CAZ, ceftazidime; CIP, ciprofloxacin; FLU, fluconazole; ITR, itraconazole; P, penicillin; TZP, piperacillin/tazobactam; VOR, voriconazole.
Adverse events
With regard to vancomycin, ciprofloxacin, and voriconazole toxicity, 6 patients (24%) had hypersensitivity reactions, red man syndrome, during initial vancomycin prophylaxis. The reactions were well prevented by slowing the injection rates and antihistamine premedication during the following prophylaxis courses. There were 12 episodes (6%) of elevated transaminase levels, 10 (5%) of grade 1 and 2 (1%) of grade 2, reported in 8 patients while no renal toxicity or hypokalemia were observed during antimicrobial prophylaxis.
Discussion
There are several findings from the current study. First, prophylaxis treatment effectively decreased the occurrence of BSIs in pediatric AML patients receiving induction chemotherapy, and both BSIs and IFIs in post-remission HD chemotherapy. Second, prophylaxis treatment significantly reduced the episodes of FN as well as death related to life threatening infections during chemotherapy. Third, exposure to antibiotics, especially amikacin and carbapenem, and antifungal agents such as Caspofungin and amphotericin-B during the prophylaxis period were significantly lower than those for the treatment of any infections during the preprophylaxis period. Fourth, we demonstrated the degree of amikacin susceptibility against E. coli, K. pneumoniae, E. cloacae, P. aeruginosa, and C. freundii was improved during the prophylaxis period. Furthermore, although the carbapenem exposure declined during the prophylaxis period, the resistance to imipenem among E. cloacae and A. baumannii were more common. The rate of multidrug-resistant Enterococci was increased remarkably during the prophylaxis period. Finally, in this comparative historical study, the overall outcome in pediatric AML patients was improved significantly during the prophylaxis period in comparison to the preprophylaxis period.
The advancement of supportive care measures have, doubtlessly, contributed significantly to the improvement in outcomes for children with AML patients. Studies conducted by the AML-BFM group and the St. Jude AML trials showed that decreased treatment-related mortality in AML patients improved over successive clinical trials in the 1990s and 2000s3,18. Infectious complications remain a major cause of morbidity and mortality for pediatric AML patients who are at particular risk of Viridians Group Streptococci (VGS), GNB, and IFI19. A high incidence of VGS and GNB infections in pediatric AML6,19 administration of antibiotics prophylaxis was reasonable to reduce the rates of BSI during intensive treatment5,20. In the study institution, several infection control practices other than antimicrobials prophylaxis have been added over time to try to reduce the incidence of infection. First, the pediatric intensive care unit was renovated in 2008 and the children’s oncology ward in 2013 since the hospital building was more than 30 years old. Second, a training program for oncology clinical nurse specialists has been held every 2 years since 2007. Third, a low-bacterial diet was started to provide for children with cancer receiving chemotherapy during hospitalization. These practices might be contributed to the reduction infection rates of children with cancer undergo chemotherapy.
Currently, there are no comparative studies of prophylaxis with vancomycin in children with AML who underwent chemotherapy. Kurt et al. reported that prophylaxis with vancomycin-containing regimens markedly reduced the odds of VGS sepsis by 99% relative to no prophylaxis20. The present report demonstrated the cumulative incidence of gram-positive BSI was 5% of chemotherapy courses in pediatric AML without vancomycin prophylaxis compared to 1% for patients with prophylaxis, and the reduction in rates of GPB BSI was significant in the prophylaxis period.
Regarding the adverse effects of vancomycin during the period of vancomycin prophylaxis, only minimal elevated transaminase levels and no nephrotoxicity were found. The results of minimal hepatic and renal toxicity during vancomycin prophylaxis in pediatric AML were similar to one recent report21. Compared with the therapeutic dose of vancomycin, the rate of nephrotoxocity was 8–20% as vancomycin troughs was maintained between 10 and 15 mg/l22. We believe that routine therapeutic vancomycin level monitoring is not necessary during the use of vancomycin prophylaxis. Although the susceptibility of vancomycin to two most common GPB, coagulase-negative staphylococcus and staphylococcus aureus, were no different during the prophylaxis period, the susceptibility of many classes of antibiotics including vancomycin, tecoplanin, linezolid, and ampicillin/sulbactam to Enterococcus species were significantly reduced during the prophylaxis period in the study institution. The trend of increasing the proportion of Vancomycin-resistant Enterococcus was noted so we start to omit prophylaxis with vancomycin for AML patients receiving MD chemotherapy in order to reduce vancomycin exposure in patients with low infection risk. Further research should aim to compare the efficacy, toxicity, and antibiotics susceptibility of vancomycin-based prophylactic regimen and alternative regimens in independent cohorts.
Development of antimicrobial resistance has increased dramatically over the past few years and has become a great public health problem worldwide. GNB such as E. coli and K. pneumoniae have displayed increasing resistance to second-, third-, or fourth-generation cephalosporins for neonatal and infant sepsis, ranging from 23 to 51%, in certain developing countries23,24. Moreover, the epidemiology of carbapenem-resistant enterobacteriaceae had increased from 1 to 12% in US hospitals25 with a 50% associated mortality rate26 between 2000 and 2010. Therefore, we put forward a report on changes in the antibiotic susceptibility of bacteria at the study institution and analyze the impact of antimicrobial prophylaxis strategies on antibiotic susceptibility of bacteria. This has not been previously reported in detail in pediatric AML patients under antimicrobial prophylaxis8,9. In the present study, the number of days used for amikacin or carbapenam was significantly reduced in children with AML during the prophylaxis period. Several possible reasons are as follows: first, empiric or therapeutic amikacin was used less frequently due to the concern of its nephrotoxicity. Second, episodes of FN or BSI were occurred less frequently, thus reducing the number of days used for carbapenem. Although the number of days used for carbapenem was reduced during the prophylaxis period, some bacteria, such as E. cloacae or A. baumannii, observed were less sensitive to carbapenem at study institution. This may be due to the fact that the study institution is one general children’s hospital that treats children with different kinds of acute or chronic illnesses. Late-line antibiotics were used frequently for the treatment of severe bacterial infections. Therefore, the sensitivity of bacteria to carbapenem has gradually reduced in recent years. We have tried to maintain carbapenam resistance by administering other antimicrobials (eg. cephalosporin and aminoglycoside) when patients are found to have GNB BSI and reserving carbapenam as a second-line antibiotic.
Among 16 episodes of BSI during the prophylaxis period, 13 (81%) were breakthrough BSI (bBSI). Susceptibility of 13 bBSI to cefuroxime, ceftazidime, amikacin, piperacillin/tazobactam, imipenem, and ciprofloxacin were 43, 50, 93, 64, 93%, and 0%, respectively, in the present study. Cefuroxime and amikacin were administered empirically for FN during ciprofloxacin prophylaxis because their susceptibility rate to major GNB was more than 90% at the study institution. For patients with renal insufficiency, amikacin was not administered and piperacillin/tazobactam was used to preserve their renal function. Carbapenam was reserved for patients with bBSI only in order to have effective therapy for GNB and maintaining its resistance.
Since ciprofloxacin has been used as a prophylaxis in neutropenic patients, the susceptibility of ciprofloxacin to the most common 6 GNB at the study institution from 2002 to 2019 was investigated. A part of the antimicrobial susceptibility result has been reported previously5 and this study included additional patients with longer follow-up. Our previous 3-year study5 reported the susceptibility of ciprofloxacin to E. coli, K. pneumoniae, P. aeruginosa, and Serratia marcescens significantly increased during the prophylaxis period, whereas no significant changes in susceptibility to ciprofloxacin between the preprophylaxis and prophylaxis periods in the present study. Ciprofloxacin was selected continuously as a prophylactic agent because of its susceptibility rate to major GNB was around 80–90% at the study institution. However, one global surveillance study demonstrated that fluoroquinolone resistance rates increased in the past years in almost all bacterial species27. We have proposed several strategies to preserve ciprofloxacin susceptibility: first, administering other antimicrobials (eg. cephalosporin) when patients have GNB infections and reserving ciprofloxacin as a second-line antibiotic; second, administering G-CSF in patients with profound neutropenia after intensive chemotherapy in order to reduce the neutropenic period, therefore, ciprofloxacin exposure might be reduced; third, antimicrobial prophylaxis with cefepime instead of ciprofloxacin for neutropenic patients requiring secondary prophylaxis.
In conclusion, the current study provides new information by comparing prophylaxis with antimicrobials in children with AML without prophylaxis. All patients were treated according to a single protocol at a single institution and were provided with standard supportive care. For patients undergoing intensive chemotherapy, antimicrobials prophylaxis was found to significantly reduce the rate of BSIs and IFIs. Prophylaxis also reduced the episodes of FN and the mortality caused by severe infections. The exposure of amikacin, carbapenem, caspofungin, and amphotericin-B were significantly reduced and overall survival was improved significantly during the prophylaxis period. These results support the importance of antibiotics and antifungal prophylaxis to prevent treatment related morbidities, especially BSIs and IFIs, for children with AML.
Data availability
The data are available on request from the authors.
References
Zwaan, C. M. et al. Collaborative efforts driving progress in pediatric acute myeloid leukemia. J. Clin. Oncol. 33, 2949–2962. https://doi.org/10.1200/jco.2015.62.8289 (2015).
Rubnitz, J. E. et al. Clofarabine can replace anthracyclines and etoposide in remission induction therapy for childhood acute myeloid leukemia: The AML08 multicenter, randomized phase III trial. J. Clin. Oncol. 37, 2072–2081. https://doi.org/10.1200/jco.19.00327 (2019).
Alexander, T. B. et al. Decreased relapsed rate and treatment-related mortality contribute to improved outcomes for pediatric acute myeloid leukemia in successive clinical trials. Cancer 123, 3791–3798. https://doi.org/10.1002/cncr.30791 (2017).
Rasche, M. et al. Successes and challenges in the treatment of pediatric acute myeloid leukemia: A retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia 32, 2167–2177. https://doi.org/10.1038/s41375-018-0071-7 (2018).
Yeh, T. C. et al. Severe infections in children with acute leukemia undergoing intensive chemotherapy can successfully be prevented by ciprofloxacin, voriconazole, or micafungin prophylaxis. Cancer 120, 1255–1262. https://doi.org/10.1002/cncr.28524 (2014).
Sung, L. et al. Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood 110, 3532–3539. https://doi.org/10.1182/blood-2007-05-091942 (2007).
Lehrnbecher, T. et al. Infectious complications in pediatric acute myeloid leukemia: Analysis of the prospective multi-institutional clinical trial AML-BFM 93. Leukemia 18, 72–77. https://doi.org/10.1038/sj.leu.2403188 (2004).
Inaba, H. et al. Feasibility, efficacy, and adverse effects of outpatient antibacterial prophylaxis in children with acute myeloid leukemia. Cancer 120, 1985–1992. https://doi.org/10.1002/cncr.28688 (2014).
Alexander, S. et al. Effect of levofloxacin prophylaxis on bacteremia in children with acute leukemia or undergoing hematopoietic stem cell transplantation a randomized clinical trial. JAMA 320, 995–1004. https://doi.org/10.1001/jama.2018.12512 (2018).
Fisher, B. T. et al. Effect of caspofungin vs fluconazole prophylaxis on invasive fungal disease among children and young adults with acute myeloid leukemia a randomized clinical trial. JAMA 322, 1673–1681. https://doi.org/10.1001/jama.2019.15702 (2019).
Gafter-Gvili, A. et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst. Rev. 1, CD004386. https://doi.org/10.1002/14651858.CD004386.pub3 (2012).
Liang, D. C. et al. Improved treatment results for childhood acute myeloid leukemia in Taiwan. Leukemia 20, 136–141. https://doi.org/10.1038/sj.leu.2403979 (2006).
Yeh, T. C. et al. The development of a novel protocol for the treatment of de novo childhood acute myeloid leukemia in a single institution in Taiwan. J. Pediatr. Hematol. Oncol. 29, 826–831. https://doi.org/10.1097/MPH.0b013e31815a05aa (2007).
Troke, P. F., Hockey, H. P. & Hope, W. W. Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Antimicrob. Agents Chemother. 55, 4782–4788. https://doi.org/10.1128/AAC.01083-10 (2011).
National Cancer Institute. Common terminology criteria for adverse events v5.0 (CTCAE). NCI, NIH, DHHS. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (2017).
Centers for Disease Control and Prevention. Bloodstream infection event (central line-associated bloodstream infection and non-central line-associated bloodstream infection). http://www.cdc.gov/nhsn/PDFs/pscManual/4PSC_CLABScurrent.pdf (2017).
Pauw, B. D. et al. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group; National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46, 1813–1821. https://doi.org/10.1086/588660 (2008).
Creutzig, U. et al. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: Analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J. Clin. Oncol. 22, 4384–4393. https://doi.org/10.1200/JCO.2004.01.191 (2004).
Gamis, A. S. et al. Alpha hemolytic streptococcal infection during intensive treatment for acute myeloid leukemia: A report from the Children’s cancer group study CCG-2891. J. Clin. Oncol. 18, 1845–1855. https://doi.org/10.1200/JCO.2000.18.9.1845 (2000).
Kurt, B. et al. Prophylactic antibiotics reduce morbidity due to septicemia during intensive treatment for pediatric acute myeloid leukemia. Cancer 113, 376–382. https://doi.org/10.1002/cncr.23563 (2008).
Sun, Y. et al. Adverse effects of intravenous vancomycin-based prophylaxis during therapy for pediatric acute myeloid leukemia. Antimicrob. Agents Chemother. 62, e01838-17. https://doi.org/10.1128/AAC.01838-17 (2018).
van Hal, S. J. et al. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob. Agents Chemother. 57, 734–744. https://doi.org/10.1128/AAC.01568-12 (2013).
Li, J. Y. et al. Identification and antimicrobial resistance of pathogens for neonatal septicemia in China—A meta-analysis. Int. J. Infect. Dis. 71, 89–93. https://doi.org/10.1016/j.ijid.2018.04.794 (2018).
Downie, L. et al. Community-acquired neonatal and infant sepsis in developing countries: Efficacy of WHO’s currently recommended antibiotics—Systematic review and meta-analysis. Arch. Dis. Child. 98, 146–154. https://doi.org/10.1136/archdischild-2012-302033 (2013).
Kuehn, B. M. “Nightmare” bacteria on the rise in US hospitals, long-term care facilities. JAMA 309, 1573–1574. https://doi.org/10.1001/jama.2013.2922 (2013).
Freifeld, A. G. et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin. Infect. Dis. 52, e56-93. https://doi.org/10.1093/cid/cir073 (2011).
Dalhoff, A. Global fluoroquinolone resistance epidemiology and implictions for clinical use. Interdiscip. Perspect. Infect. Dis. 2012, 976273. https://doi.org/10.1155/2012/976273 (2012).
Acknowledgements
The authors thank Mr. William Johnson for editing the text.
Author information
Authors and Affiliations
Contributions
T.-C.Y. Data acquisition, quality control of data and algorithms, data analysis and interpretation, statistical analysis, writing–initial draft, and writing–review and editing. J.-Y.H., T.-H.H., C.-H.L., F.-J.S., and H.-M.H. Data acquisition, quality control of data and algorithms, data analysis and interpretation. H.-C.L. Study concept and design, data acquisition, quality control of data and algorithms, data analysis and interpretation, statistical analysis, writing—initial draft, and writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yeh, TC., Hou, JY., Huang, TH. et al. Effectiveness and antimicrobial susceptibility profiles during primary antimicrobial prophylaxis for pediatric acute myeloid leukemia. Sci Rep 11, 21142 (2021). https://doi.org/10.1038/s41598-021-00725-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-00725-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.