RET Inhibitors Show Promise for Chinese Patients With RET Fusion–Positive NSCLC
Data continue to support the use of RET inhibitors is patients with RET fusion-positive non–small cell lung cancer.
RET inhibitors pralsetinib Gavreto) and selpercatinib (Retevmo) have already demonstrated significant efficacy in treating a broad patient population with tumors harboring RET fusions and mutations. Indeed, both RET inhibitors are approved for use in the United States for the treatment of patients with metastatic RET fusion–positive non–small cell lung cancer (NSCLC).
However, only pralsetinib has been approved by the National Medical Products Administration of China as a treatment for patients with locally advanced or metastatic RET fusion–positive NSCLC following platinum-based chemotherapy.1
In findings presented during the International Association for the Study of Lung Cancer 2021 World Conference on Lung Cancer from 2 separate trials, pralsetinib and selpercatinib both showed significant efficacy in the treatment of Chinese patents with advanced RET fusion–positive NSCLC.2,3
Pralsetinib
In data from the Chinese cohort of the global phase 1/2 ARROW study (NCT03037385), pralsetinib demonstrated promising results in patients with advanced RET fusion–positive NSCLC regardless of previous therapies.2
“Pralsetinib is a promising type of treatment with rapid and durable clinical activity in Chinese patients with RET fusion–positive non–small cell lung cancer regardless of the previous treatment. And the safety profile is manageable and the tolerable,” said lead study author Qing Zhou, MD, of the Guangdong Lung Cancer Institute in China.
There were 63 patients enrolled in the Chinese cohort of the study; 33 had prior platinum-based chemotherapy treatment, and 30 had no prior systemic treatment.
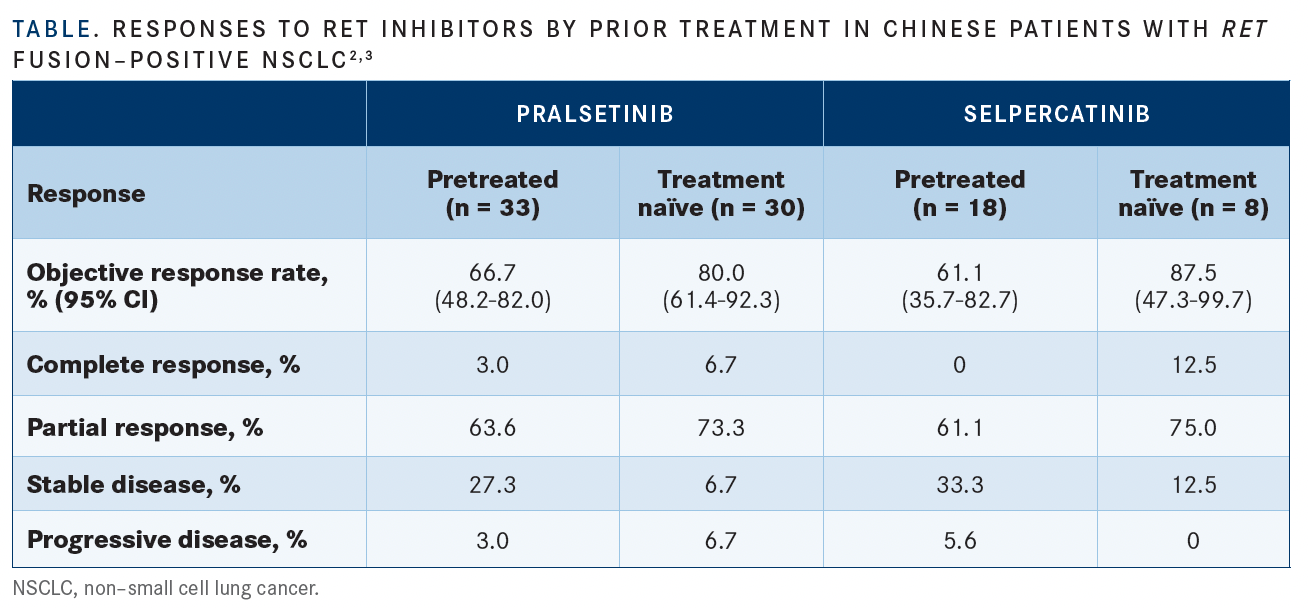
Those who had prior chemotherapy achieved an overall response rate (ORR) of 66.7% (n = 22; 95% Cl, 48.2%-82.0%) at a median follow-up of 17.0 months (range, 16.3-18.1); 3% (n = 1) had a complete response (CR), and 63.6% (n = 21) had a partial response (PR) (TABLE2,3).
Researchers reported that the median time to first response among this pretreated patient cohort was 1.87 months (range, 1.7-3.8). Six-month and 9-month duration of response (DOR) rates were 77.3% (95% Cl, 59.8%-94.8%) and 50.0% (95% Cl, 29.1%- 70.9%), respectively. Treatment duration was a median of 14.65 months (range, 0.9-20.0).
In the group of patients who had not received prior therapy, the ORR was 80% (n = 4; 95% Cl, 61.4%-92.3%), of which 6.7% (n = 2) experienced a CR and 73.3% (n = 22) experienced a PR. Median follow-up for these patients was 8.2 months (range, 7.1-8.6), and the median treatment duration was 7.13 months (range, 0.5-14.0). Median time to first response among the group was 1.87 months (range, 1.7-3.8). DOR rates were 76.7% (95% Cl, 55.6%-97.8%) and 38.3% (95% Cl, 0.0%-92.5%) at the 6- and 9-month marks, respectively.
Of note, 68.18% (n = 15) of responders who were on platinum-based chemotherapy previously and 79.17% (n = 19) of those without prior treatment remain on the treatment.
“I think pralsetinib should be a new standard of care for both Chinese patients and other global patients with RET fusion–positive advanced non–small cell lung cancers,” Zhou said.
Data from the study reported that pralsetinib was well tolerated in the Chinese patient cohort with a manageable safety profile. Treatment-emergent adverse events (TRAEs) were experienced in all 68 patients, 7 of which discontinued treatment due to a TRAE.
“I think compared to other targeted treatment, we should pay more attention to the hematological toxicities,” Zhou noted, highlighting neutropenia, anemia, and decreased platelet count as some of the hematological AEs to look out for.
Common any-grade TRAEs included increased aspartate aminotransferase (AST) levels (80.9%), decreased white blood cell count (60.3%), increased alanine aminotransferase (ALT) levels (57.4%), increased blood creatine phosphokinase levels (45.6%), hypertension (35.3%), increased blood creatine levels (29.4%), increased conjugated bilirubin levels (27.9%), constipation (27.9%), increased γ-glutamyltransferase levels (27.9%), increased blood alkaline phosphatase levels (26.5%), malaise (25.0%), increased blood bilirubin levels (23.5%), and hypocalcemia (20.6%).
Grade 3 or 4 TRAEs that occurred in 5% or more were decreased lymphocyte counts (5.9%), leukopenia (5.9%), and hypophosphatemia (11.8%).
Selpercatinib
Selpercatinib demonstrated robust and durable efficacy with a favorable safety profile in Chinese patients with advanced, RET fusion– positive NSCLC in the phase 2 LIBRETTO-321 trial (NCT04280081).3
Among a subset of patients with central lab–confirmed RET-fusion status, considered the primary efficacy analysis set (PAS; n = 26), the Independent Review Committee (IRC)–assessed ORR observed with selpercatinib was 69.2% (95% CI, 48.2%-85.7%), with 94.4% of responses ongoing at a median follow-up of 9.7 months. In all patients with NSCLC (n = 47), the IRC-assessed ORR achieved with the agent was 66.0% (95% CI, 50.7%-79.1%), with 96.8% of responses ongoing at a median follow-up of 10.3 months.
“These findings were consistent with previous data in global and East Asian populations from [the phase 1/2] LIBRETTO-001 trial [NCT03157128], suggesting that selpercatinib is a promising treatment option for Chinese patients with RET fusion–positive NSCLC,” lead study author Shun Lu, MD, PhD, said. Lu is a professor at Shanghai Chest Hospital at Jiao Tong University and chief of the Shanghai Lung Cancer Center in China.
The ongoing, open-label, multicenter, phase 2 LIBRETTO-321 trial being conducted in China is examining the safety and efficacy of selpercatinib in patients 18 years or older with treatment-naïve or pretreated locally advanced or metastatic tumors.
A total of 77 patients were enrolled in the study and divided into 3 cohorts. Cohort 1 (n = 30) was comprised of patients with advanced RET fusion–positive solid tumors who had progressed on or were intolerant to 1 or more prior standard first-line therapies or those who had declined or were not suitable to receive standard frontline therapy. Cohort 2 (n = 26) enrolled patients with advanced RET-mutant medullary thyroid cancer who had or had not received previous systemic therapy.
Lastly, patients enrolled to cohort 3 (n = 21) included those with advanced RET-altered solid tumors that met the requirements for cohorts 1 or 2 but did not have measurable disease, those with a RET-altered solid tumors or a RET alteration/activation that did not meet the criteria for cohorts 1 or 2, and those who were circulating tumor DNA positive for a RET alteration that was not known to be present in their tumor.
The analysis included 47 patients with NSCLC and a subset of 26 patients who had NSCLC confirmed via a central laboratory to have RET fusion status (PAS).
Study participants received oral selpercatinib at a twice-daily dose of 160 mg, administered in 28-day cycles. Treatment was administered until either disease progression, intolerable toxicity, withdrawn consent, or death. Notably, patients could continue the study regimen beyond progression if continued benefit was observed.
The primary end point of the study was ORR per RECIST v1.1 criteria and IRC assessment. Secondary end points comprised ORR per RECIST v1.1 criteria and investigator assessment, DOR, central nervous system (CNS) ORR, CNS DOR, clinical benefit rate, time to relapse (TTR), time to best response, progression-free survival, overall survival, pharmacokinetics, and safety.
Across the 2 groups, patients had a median age of 53 years (range, 26-72), and the majority were female (55.9%) and had an ECOG performance status of 1 (86.8%). The median number of prior treatments received was 2 (range, 0-9), and most patients had previously undergone treatment with platinum-based chemotherapy (68.9%). Additionally, 33.5% of patients were treatment naïve, 33.5% had CNS metastases, and 91.5% had measurable disease.
Additional data showed that in the PAS subset, selpercatinib elicited an ORR of 61.1% (95% CI, 35.7%-82.7%) in those who were pretreated (n = 18) vs 87.5% (95% CI, 47.3%-99.7%) in those who were treatment naive (n = 8) (TABLE2,3). In the all-NSCLC group, the ORRs experienced with the agent in those who were pretreated (n = 36) and treatment naive (n = 11) were 58.3% (95% CI, 40.8%-74.5%) and 90.9% (95% CI, 58.7%- 99.8%), respectively.
In the PAS subset, 1 patient achieved a CR to treatment (3.8%), and 17 achieved a PR (65.4%); the stable disease rate was 26.9% (n = 7). In the all-NSCLC population, 3 patients achieved a CR (6.4%), 28 achieved a PR (59.6%), and the stable disease rate was 29.8% (n = 14).
Additionally, 80% of patients with measurable CNS lesions at the time of enrollment achieved an IRC-assessed confirmed intracranial response at a median follow-up of 9.3 months.
The median TTR for both groups was 1.8 months, and the median DOR had not yet been reached in either group. At data cutoff, 94.4% of responding patients in the PAS subgroup continued to respond to treatment vs 96.8% in the all-NSCLC population.
In a safety population comprised of 77 patients, 97.4% experienced at least 1 treatment-emergent AE (TEAE) of any grade, and 97.4% reported a TRAE of any grade. The most common grade 3 or higher TEAEs experienced with selpercatinib included hypertension (19.5%), increased ALT levels (15.6%), and increased AST levels (15.6%). Frequently reported grade 3 or higher TRAEs included hypertension and increased ALT and AST levels (15.6% each).
TEAEs resulted in treatment discontinuation in 5.2% of patients (n = 4), with 3.9% (n = 3) considered related to selpercatinib. Moreover, 32.5% (n = 25) of patients required dose reductions. Additionally, 1 patient died because of grade 5 acute pancreatitis, although this effect was not considered related to treatment with selpercatinib.
Selpercatinib is currently being examined as initial treatment in patients with advanced or metastatic RET fusion–positive NSCLC as part of the phase 3 LIBRETTO-431 trial (NCT04194944).
REFERENCES:
1. CStone announces new drug approval of Gavreto (pralsetinib) as first selective RET inhibitor in China, providing a new therapy for a subset of non-small cell lung cancer patients. News release. CStone Pharmaceuticals. March 24, 2021. Accessed October 1, 2021. https:// bit.ly/39W4P88
2. Zhou Q, Wu Y-L, et al. Efficacy and safety of pralsetinib in Chinese patients with advanced RET fusion+ non-small cell lung cancer. Presented at: 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. Abstract M02.02.
3. Lu S, Cheng Y, Huang D, et al. Efficacy and safety of selpercatinib in Chinese patients with RET fusion-positive non-small cell lung cancer: a phase 2 trial. Presented at: International Association for the Study of Lung 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. Abstract MA02.01.

Savona Discusses First-Line JAK Inhibition for Patients With Myelofibrosis at Risk of Anemia
April 17th 2024During a Case-Based Roundtable® event, Michael Savona, MD, and participants discussed the case of a patient with myelofibrosis and moderate anemia receiving JAK inhibitor therapy.
Read More