A Food and Drug Administration (FDA) advisory panel voted to approve a second dose of the Johnson & Johnson COVID-19 vaccine Friday.
A second dose of the vaccine has been approved under emergency use authorization for people 18 and older at least two months after they receive the first dose.
There was concern among the Vaccine and Related Biological Products Advisory Committee over the small sample size in the data presented from Johnson & Johnson and the lack of review from the FDA.
FDA's Dr. Doran Fink said "public interest and sense of urgency" led to a rushed hearing and therefore "we were not able to conduct an independent verification of their data sets or their analyses or conduct our own analyses."
The committee also said the Johnson & Johnson vaccine is a two-dose vaccine, based on data presented on the efficacy of the first dose compared to other mRNA vaccines and the proposed booster dose.
"I think it's better as a two-dose vaccine," Dr. Paul Offit said. "It would be hard to recommend this as a single-dose vaccine at this point."
The voting question was revised as well. The committee voted to approve the booster dose two months after the primary series, refusing to consider a six-month interval between first and second doses.
Some committee members wanted to revise the question to say "additional shot" instead of "booster" to reflect their view that the Johnson & Johnson shot should have always been a two-dose vaccine.
The 19-strong committee voted unanimously in favor of a Moderna COVID-19 vaccine booster shot yesterday after several lengthy presentations on data that shows how an extra dose of vaccine can counteract the waning effectiveness of current shots over time.
Despite all members voting "yes" after lengthy presentations, many members still had concerns over the data they saw from Moderna and the Israel Health Ministry, worried that it did not reflect a fully-rounded picture, and answer questions about the necessity of the boosters - and who should get them.
The live updates for this blog have ended.
Bill for vaccine mandate exemptions passes West Virginia legislature
A bill to allow certain medical and religious exemptions to COVID-19 vaccine mandates passed West Virginia's Republican-led House of Delegates Friday.
The proposal would allow employees to evade punishment from current or prospective employers for pursuing exemptions from vaccine mandates.
"I don't believe that really and truly we should just throw our freedoms and our rights in a trash can," Governor Jim Justice said in a news conference.
Under the bill, a doctor or nurse can give signed documentation that an employee has a physical condition preventing them from receiving the COVID-19 vaccine. Employees can also present a notarized certification for religious exemptions.
"If my employer wants to require something, fine. You can still require it. But you're going to recognize these exemptions. They're legitimate and they exist," Raleigh County Republican Brandon Steele, chairman of the House government organization committee, said.
The bill is pending in the state Senate.
U.S. set to discuss Pfizer vaccines for children under 12 in coming weeks
The U.S. officials will discuss the smaller-dose version of the Pfizer vaccine for children under 12 in the coming weeks.
Pfizer/BioNTech sent data to the U.S. Food and Drug Administration (FDA) with a request to approve their COVID-19 vaccine to children ages 5 to 11.
The Centers for Disease Control and Prevention (CDC) sent a seven-page document to help states and cities set up expanded vaccination programs.
Pfizer/BioNTech submitted the same data to the European Medicines Agency Friday.
The study tested their vaccine in more than 2,200 children ages 6 months to 11 years. The children in this study received a lower dose than what's normally given to adults.
The results showed a "strong immune response" in the children and found that the vaccine was safe, the companies said in a statement.
An FDA expert advisory panel is scheduled to publicly debate the evidence at a meeting in late October. If the FDA authorizes vaccines for children, a CDC advisory panel would discuss the data in early November and offer its recommendation to the CDC.
Johnson & Johnson science chief "pleased" with FDA advisory committee decision
Johnson & Johnson's science chief responds to the FDA advisory committee recommendation to grant emergency use authorization to its vaccine booster dose.
"We are pleased with the unanimous vote by the U.S. FDA Vaccines and Related Biological Products Advisory Committee to recommend a booster of the Johnson & Johnson vaccine," Dr. Pual Stoffels said in a statement.
The @US_FDA Advisory Committee voted unanimously to recommend the FDA grant Emergency Use Authorization for a #booster dose of the Johnson & Johnson #COVID19 #vaccine.
— Johnson & Johnson (@JNJNews) October 15, 2021
The Janssen COVID-19 Vaccine has not been approved/licensed by the FDA. https://t.co/Xnh5QXScdQ pic.twitter.com/46O7xOUnmj
More people are getting booster shot than first doses in Indiana
More people in Indiana have received COVID-19 booster shots than new doses of vaccinations in the last month.
According to data from the Indiana Health Department vaccine tracker, 60 percent of the shots administered were booster shots to people who were fully vaccinated.
While the demand for Pfizer boosters has increased following the FDA's approval in September, the amount of first or second doses received in Indiana has hit its lowest level since the vaccine became widely available last year, the Associated Press reported.
About 49 percent of Indiana's residents are fully vaccinated, according to the Centers for Disease Control and Prevention.
Committee discusses mix and match booster shot study
The committee is now discussing general observations that can be made regarding the data on heterologous boosters presented by the National Institutes of Health (NIH) from their Mix and Match Booster Study.
Dr. Kirsten Lyke from the University of Maryland School of Medicine presented the FDA panel with data from a study on mixing booster shots.
Lyke said the NIH study is adaptive and will include more data on mix and match with the Moderna and Johnson & Johnson booster doses.

The study found that all of the shots resulted in a "boost" of antibodies for recipients. Moderna primary or booster dose recipients had the best antibody responses and there were limited adverse reactions.
More research is needed to determine the correlations between antibody response and protection against infection and severe disease.
The study concluded that heterologous, a mix of vaccine brands, led to a similar or higher antibody response compared to their respective homologous booster responses.
It also found that mRNA vaccine boosters, like Moderna and Pfizer, resulted in a higher level of antibodies in the 28 days after the booster was given.
Committee disputes wording of voting question
The committee is asking to reword the voting question, as many members believe an additional Johnson & Johnson dose is more of a second shot than a booster.
The committee is going to adjust the voting question to decide if an additional Johnson & Johnson should be given at least two months after the first dose.
The committee will only be voting on the first question: if the data support the safety and effectiveness of the Johnson & Johnson as a booster dose for people over the age of 18 at least two months after the first dose is given.
Some committee members want to revise the question to say "additional shot" instead of "booster."
Here's the updated voting question: pic.twitter.com/jJm9G9RfWa
— David Lim (@davidalim) October 15, 2021
Committee member says Johnson & Johnson is "better as a two-dose vaccine"
Several members of the committee are questioning if the proposed Johnson & Johnson booster dose is more of a second dose.
Johnson & Johnson is presenting the second dose as a booster to the one-shot vaccine and is not looking to change its authorization to a two-dose vaccine.
Committee member Dr. Eric Rubin, editor-in-chief of The New England Journal of Medicine, said the company should focus on a booster dose two months after the first dose.
"If the vaccine isn't adequate, then it should be boosted in everybody," Rubin said. He noted that the data for a six-month interval is slim.
"I'm not sure why you're asking for an indication that would apply to millions of patients with a data set that includes 17 patients."
Dr. Paul Offit, a vaccine expert from Children's Hospital of Philadelphia, agreed with Rubin and said the Johnson & Johnson vaccine "was always a two-dose vaccine."
"I think it's better as a two-dose vaccine," he said, "it would be hard to recommend this as a single-dose vaccine at this point."
CDC data suggests Johnson & Johnson vaccine has low hospitalization efficacy
During the commission discussion, FDA's Dr. Peter Marks suggests other show shows Johnson & Johnson vaccine has a lower effectiveness for emergency room visits and hospitalizations compared to mRNA vaccines.
The CDC's Amanda Cohn supported that notion, saying the effectiveness of protection with a single dose of the Johnson & Johnson vaccine is not the same as the protection from the Moderna or Pfizer vaccines.
"In our hospitalization network, so in our active surveillance that looks at vaccine effectiveness in hospitalized individuals, we demonstrated that the Janssen vaccine was only 68 percent effective against hospitalization, and this is in adults greater than 18 years of age without immunocompromising conditions, which is both lower than what we saw from that real-world effectiveness presentation and it is also substantially lower than the mRNA vaccines are against hospitalization even with the waning," Cohn said.
"Additionally there was some other data to suggest that real-world effectiveness is hovering more in the 50 percent to 60 percent [range], and this is from some data from a different surveillance system," she added.
FDA suggests "there is more data that is out there" on the Johnson & Johnson booster
The FDA suggests Johnson & Johnson is not telling the committee the whole story on booster shot efficacy.
FDA's Dr. Peter Marks told the committee Johnson & Johnson may not be telling the whole story.
"There is more data that is out there than what we are seeing," he said. "There are data suggesting the effectiveness of the vaccine is actually less robust than the company's presentation here."
Some of the data sets were very small. One of the studies submitted to the FDA looking into the efficacy of the Johnson & Johnson booster after six months included only 17 people.
Johnson & Johnson said this study still shows robust, compelling responses. The committee seems skeptical.
FDA was unable to independently verified data on Johnson & Johnson booster shot
The FDA was not able to verify data presented on the Johnson & Johnson booster shot.
FDA's Dr. Doran Fink said the "public interest and sense of urgency" in presenting a second dose based on data for the rush of the hearing.
He said data sets on the Johnson & Johnson booster shot were not submitted to the FDA until recently and therefore the agency could not conduct its usual review process.
"As a consequence of the review time, we were not able to conduct an independent verification of their data sets or their analyses or conduct our own analyses," he added.
During the committee hearing Friday, FDA's Rachel Zhang said "except for the immunogenicity assessments of the six-month booster interval," the datasets for the studies "were not submitted in sufficient time for FDA to conduct an independent review to verify Johnson & Johnson analyses."
The FDA was also unable to independently verify Johnson & Johnson's safety data.
Johnson & Johnson says its booster raises efficacy of its first dose
Johnson & Johnson said while mRNA vaccines, like Moderna and Pfizer/BioNTech, create higher neutralizing antibodies right after the shot, Johnson & Johnson's shot uses more traditional virus-based technology and creates a robust and more stable response that lasts at least eight months.
Johann Van Hoof, managing director of Janssen Vaccines, is against mixing vaccine brands when administering boosters. He pushed for homologous booster doses, boosting the first Johnson & Johnson shot with a second Johnson & Johnson shot, believing Johnson & Johnson protection is durable.
However, FDA reviewers pointed out the efficacy for the single-dose Johnson & Johnson vaccine has been lower than the efficacy of the other mRNA vaccines.
Johnson & Johnson's Penny Heaton noted "while the efficacy has been stable, it's been consistent." She said the vaccine's efficacy increased from about 74 percent to 94 percent when a booster dose was given after two months, highlighting the vaccines' "unique profile."
Any waning efficacy seen in its single-dose vaccine is linked to the spread of variants, not a function of time, Johnson & Johnson officials said.
This data differs from what was presented by Pfizer and Moderna.
Moderna and Pfizer's booster returned their vaccine's efficacy to normal, while Johnson & Johnson's booster increase its original dose efficacy to the level of the other brands of vaccines, according to STAT News' Helen Branswell.
Johnson & Johnson voting question stirs confusion among committee
There is confusion among the FDA advisory committee over the voting questions presented by Johnson & Johnson.
There will be two votes at this hearing.
The first vote will determine if the data support the safety and effectiveness of the Johnson & Johnson booster dose for adults 18 years of age and older two months after the primary vaccination dose.
Here is the voting questions for $JNJ booster for later. pic.twitter.com/BhaLfKtDbC
— Sarah Karlin-Smith (@SarahKarlin) October 15, 2021
The second vote is based on whether the committee voted yes or no to the first questions.
If they vote yes on the first question, the committee will next vote if extending the interval between the first dose and the booster to at least six months will result in "a more robust booster response."
If they vote no on the first question, the committee will vote if the data support a booster shot given six months after the first shot.
Anyone over the age of 18, regardless of health status, can get a Johnson & Johnson, according to the voting questions.
This criterion differs from Pfizer and Moderna booster shots, which were approved for specific groups of people. These groups including those over 65, people 18 to 65 with medical conditions that increase their risk for severe COVID-19, and people who live or work in places that expose them to a higher risk of contracting COVID-19.
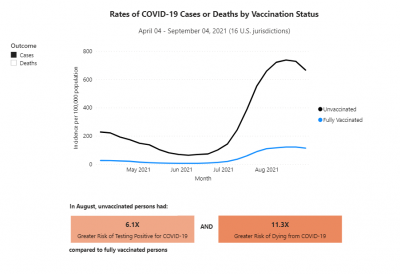
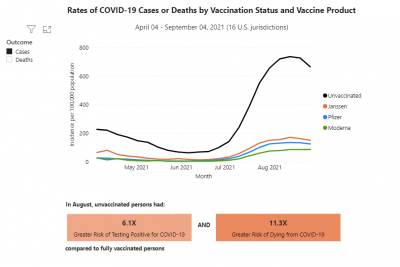
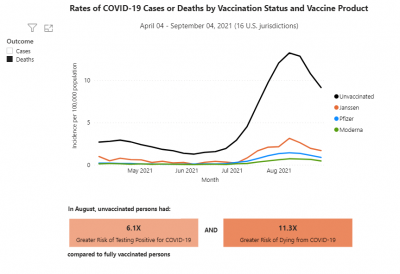
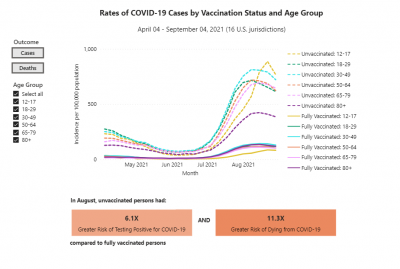
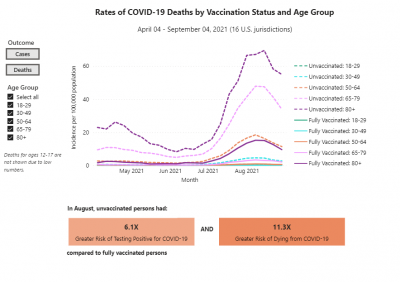
COVID-19 rates and deaths by vaccine status, brand and age, per CDC
The U.S. Centers for Disease Control and Prevention (CDC) released data showing the differences in COVID-19 cases and deaths among vaccinated and unvaccinated Americans from April 2021 to September 2021.
This pair of graphs shows the rate of cases and then the rate of deaths between unvaccinated and fully vaccinated people.

This pair of graphs shows the rate of cases and then the rate of deaths by vaccine status and vaccine brand.

This pair of graphs shows the rate of cases and then the rates of deaths by vaccination status and age group.

U.S. to lift travel restrictions from November 8
White House Assistant Press Secretary, Kevin Munoz, confirmed the reports that fully vaccinated foreign travelers will be able to come to the U.S. beginning November 8.
The plans were already confirmed but the date at which the new rules would come into effect was uncertain.
The US’ new travel policy that requires vaccination for foreign national travelers to the United States will begin on Nov 8. This announcement and date applies to both international air travel and land travel. This policy is guided by public health, stringent, and consistent. https://t.co/uaDiVrjtqi
— Kevin Munoz (@KMunoz46) October 15, 2021
Israel to ease COVID restrictions as infections and death decline
Health Ministry Director-General Nachman Ash said the country can ease more restrictions but also urged caution ahead of the winter and confirmed people won't be removing their masks indoors this season.
"As morbidity declines, we can ease [restrictions]. We are thinking of lifting more Green Pass restrictions," Ash told Radio 103FM, appearing to confirm a Thursday report that said the government was planning to push for a significant easing of COVID-19 limits.
A total of 1,325 new cases were identified nationwide yesterday - down from more than 10,000 a day at the height of the Delta variant wave in August and September.
Exasperated Idaho doctor says medical staff have 'lost the war' over COVID
"Today I'm here to tell you that we've lost the war," Dr. Steven Nemerson, chief clinical officer at Saint Alphonsus Health System, said during a press briefing on Tuesday, reports the Idaho Statesman.
The reason it is here to stay is because we cannot vaccinate enough of the public to fully eradicate the disease. And absent being able to do that ... we now need to move into the phase of recognizing that COVID is going to be a disease to be managed for the long-term future.
Idaho has faced weeks of high COVID caseloads and hospitalization rates, with over 1,000 cases each day since early September.
FULL STORY: Idaho Doctor Says 'We've Lost the War' and COVID Is 'Here to Stay' as Cases Remain High
Biden pledges to donate over 17 million vaccines to African countries
After meeting Kenyan President Uhuru Kenyatta, the president confirmed the U.S. will boost its donation to the continent after scathing criticism from the World Health Organization yesterday.

Over 43,000 possibly infectious Brits wrongly told their COVID test was negative
Testing at the Wolverhampton lab has been suspended following an investigation by the country's National Health Service. It is now contacting those affected, mostly in South West England, to ask them to take another COVID test.
Concerns were raised when people had tested positive on lateral flow tests - rapid tests used widely by British households - but had negative PCR results.
The error means potentially thousands of people infected with the virus were wrongly told to stop isolating and could have infected others.
Around 3 million U.S. workers laid off mid-pandemic stop looking for work
Around three million U.S. workers who were laid off during the pandemic have stopped looking for work and are yet to resume their job searches, new figures show. Hiring has also slowed in the past two months despite employers posting a near-record number of job openings.
Economists hoped more people would find work in September as schools reopened, lessening child care constraints, and enhanced unemployment aid ended across the U.S. However, employers added just 194,000 jobs last month - much lower than expected.
FULL STORY: 3 Million U.S. Workers Have Not Resumed Job Search After COVID Pandemic Layoffs
Biden hails 'working' pandemic plan
Our plan to accelerate a path out of this pandemic is working. pic.twitter.com/HodK4Y7glV
— Joe Biden (@JoeBiden) October 14, 2021
Over 400 million doses of COVID-19 vaccine administered - CDC
A total of 405,444,558 doses of COVID-19 vaccines have been administered as of yesterday morning and the country has distributed 490,951,045 doses in all, the agency confirmed.
The CDC said 217,953,275 people have received at least one dose so far while 188,281,747 people have been fully vaccinated.
No, Bill Clinton is not in hospital with COVID
The 75-year-old former president has been hospitalized in California for a non-heart, non-COVID-related issue, his spokesperson Angel Ureña.
On Tuesday evening, President Clinton was admitted to [University of California Irvine] Medical Center to receive treatment for a non-Covid-related infection. He is on the mend, in good spirits, and is incredibly thankful to the doctors, nurses, and staff providing him with excellent care.
After two days of treatment, his white blood cell count is trending down and he is responding to antibiotics well. The California-based medical team has been in constant communication with the President's New York-based medical team, including his cardiologist. We hope to have him go home soon.
FULL STORY: Bill Clinton Hospitalized in California With Infection
WATCH: WHO Regional Director slams richer countries which 'make a mockery of vaccine equity'
A furious World Health Organization boss in Africa, Dr. Matshidiso Moeti, slammed the world's richest countries yesterday for "making a mockery of vaccine equity" yesterday after it was revealed that less than five percent of African people are currently fully vaccinated, compared to two-thirds of American aged over 12.
U.S. Trade Official blames inability to boost vaccine production on lack of support from allies
Katherine Tai said yesterday that the U.S. remains committed to loosening intellectual property rules so COVID-19 vaccines can be produced more widely but the U.S. cannot "will something into being" without all members of the World Trade Organization offering their support too.
The Biden administration said in May that it supported waiving the intellectual property rights for COVID vaccines to boost production of the shots and Tai confirmed work was being done behind the scenes, even if it didn't appear to be "from the public vantage point".
FULL STORY: U.S. Says It Supports Vaccine Production Waiver, But All WTO Member States Must Agree
Community COVID transmission remains high despite cases dropping, CDC urges caution
New infections are "continuing to decrease but the overall level of community transmission in the U.S. is high", the agency tweeted, alongside a timeline map showing the shockingly rapid spread of the virus across the U.S. since March 2020.
#COVID19 cases are continuing to decrease, but the overall level of community transmission in the U.S. is high. 7-day average of daily new cases is 84,555, a 12.5% decrease from the previous week.
— CDC (@CDCgov) October 14, 2021
Get vaccinated as soon as you can!
More: https://t.co/iSLwhCwlZ2. pic.twitter.com/EXSxX6oRtD