Roundtable Discussion: Gadgeel Reviews Further Treatment Options for Patients With Non– Driver-Mutated NSCLC
A 59-year-old man with a history of smoking, hypertension, and osteoarthritis presented with chest pain, cough, and dyspnea.
Shirish M. Gadgeel, MD, MBBS

Shirish M. Gadgeel, MD, MBBS, the head, of the Division of Hematology and Oncology, associate Director, Patient Experience and Clinical Care at Henry Ford Health System in Detroit, MI moderated a Targeted Oncology Case-Based Roundtable event with Nasser H. Hanna, MD, the Tom and Julie Wood Family Foundation Professor of Lung Cancer Clinical Research, and professor of Medicine at Indiana University Melvin and Bren Simon Cancer Center in Indianapolis, IN.
GARG: Nowadays, any patient we see, whether they have a squamous carcinoma or an adenocarcinoma, we always do an NGS panel because we need to see if they have any targetable markers. Down the line, we also need to know the markers because we never know if we can get the patient into a clinical trial, or if they could go to a phase 1/phase 2 trial. It also helps in [treatment] of the patient. We know that [there are specific therapies for non–small cell lung cancer that is positive for mutations in the] RET and MET genes, as well as a lot of other genes. I typically use FoundationOne [testing] for NGS, and I know that a lot of [clinicians] use their in-house system or an outside laboratory, but we need to get a comprehensive panel.
As for liquid biopsies, I typically use them if I don’t have a tissue sample. I’m not using liquid biopsies up front for all patients, only when I don’t have a tissue sample or if the patient is not interested in getting a biopsy.
DUMA: All my patients get an NGS test. We try to do it in a streamlined way to help the turnaround time. My clinic is dedicated to giving patients a second opinion. I do mostly liquid biopsies [because] trying to obtain a tissue sample can take a week or 2.
I also use liquid biopsies for other tests that we can talk about later, including [to monitor] progression.
HANNA: I think how long it takes to get results back matters, depending upon the clinical situation. Also, how comprehensive do you need [the results to be] before deciding you’re not going to give targeted therapy as a first-line treatment? If I have a heavy smoker with squamous cell cancer, am I willing to wait 14 to 21 days just to find out they don’t have a targetable mutation? No, I’m not going to do that. On the other hand, if I have a nonsmoker with adenocarcinoma who does not need therapy urgently, then yes, I’m not going to initiate therapy until I fully understand their molecular status.
Now, if you look at the targetable mutations, we have 9 or 10 of them if you include things such as KRAS G12C— although that’s still under investigation—or NTRK fusions, which are very uncommon. The ones that are most likely to be found are EGFR, ALK, ROS1, and BRAF. If you’re negative across the board for those, it’s unlikely you’re going to find a targetable mutation. I want to make sure I know the results of those first. I test for all of them. I want to know if somebody has a RET fusion, I want to know if somebody has a MET exon 14 splice mutations. I even want to know if they have an NRG fusion.
And then the question really is: Are there any targetable mutations that you would not give the targeted therapy [in the first line]? I think evidence-based medicine tells us we should give targeted EGFR and ALK therapy as first-line therapy. Logic tells us that [the same applies to] MET and RET mutations and probably to HER2 mutations—although we can debate about HER2. But it’s not clear that somebody with a BRAF V600E mutation who is PD-L1 positive should be getting [a targeted therapy as a] first-line treatment, and I doubt it will be obvious for somebody with a KRAS G12C mutation.
The important thing to remember is if you only do tissue or liquid biopsies you are going to miss some mutations. And it depends on the kind of tissue testing you do. If you don’t do RNA testing, you’re going to miss some fusions as well. You will miss some ALK fusions, and you’ll miss some RET fusions. You have to be careful about what platforms you’re using. If you do both liquid and tissue biopsies, you’re going to get over 90%, with 80% concordance. But, if you only do liquid biopsies, or only tissue biopsies, you’re going to miss 1 out of 10 targetable mutations with either.
GADGEEL: I would emphasize what Dr Hanna mentioned about the RNA fusions. It’s very important to know what sort of test is being done. A lot of labs say they do NGS, but not all labs do both DNA- and RNA-based testing. So finding out what test the lab is doing is very crucial.
I also send a liquid biopsy for almost every patient. I would send a liquid biopsy for a patient [such as] this, who has multiple liver and adrenal metastases. The volume of the tumor indicates an increase in the likelihood of detecting the driver genetic alteration in liquid biopsies because those tumors tend to shed DNA into the circulation.
HANNA: [Let me say more] about the RNA [testing]. A perfect example of where you need to be careful about this is, let’s say you have [a patient who never smoked] with adenocarcinoma and your testing comes up completely negative, including no KRAS mutation. You could be missing an ALK fusion if you don’t do RNA testing. If you find something, well, fine; you probably don’t need to go the extra mile to do other things. But if you find nothing and they never smoked, I would keep looking.
GADGEEL: The NCCN [National Comprehensive Cancer Network] recommendations are consistent with what has been discussed. They do go on to say—I would like to emphasize this point—that if there is insufficient tissue to allow testing for the required markers, a repeat biopsy and/or plasma testing are suggested.1 In some patients, this is not possible. In general, in those patients where I do have a suspicion that they have a genetic alteration, and there isn’t enough tissue material, or it was not detected on plasma testing, if I’m compelled to start therapy, I usually will start with chemotherapy while I’m getting a repeat biopsy done and sending that tissue off to molecular testing.
[I start with chemotherapy] because some patients experience [treatment-related] toxicities when chemoimmunotherapy or immunotherapy alone are followed by—if you identify a genetic alteration—targeted therapy. This is due to a possible interaction between the persistent drug levels of immune checkpoint inhibitors, and the introduction of a targeted agent. A classic example is incidence of pneumonitis that is reported with osimertinib [Tagrisso] in patients who have previously received pembrolizumab [Keytruda].2 There seems to be a 90-day window [for the occurrence of these AEs]; that is, if they have received the immune checkpoint inhibitor within 90 days of initiating the targeted therapy. So it may be prudent to start off with systemic chemotherapy, if you’re compelled, while you are waiting for the tissue biopsy results.
HANNA: Completely agree, and I doubt there’s much harm in missing 1 dose of pembrolizumab if you give them [carboplatin (Paraplatin) and pemetrexed (Alimta)] while awaiting those molecular results.
KHAIRA: Do you ever have a situation where you have lung cancer where nothing turns up on the FoundationOne test twice and the tissue is too tiny to test on? Do you see that? Why would that happen?
GADGEEL: When you say that the tissue is too tiny, do you mean that the report suggested that tissue was inadequate to do the testing, or they were able to run the test and nothing showed up?
KHAIRA: The specimen was too small. We ran the FoundationOne test twice and they couldn’t get any kind of markers both times.
HANNA: You’re talking about the liquid biopsy, right? So the tissue sample was insufficient, and then you did a plasma test, and it came back with no genetic alterations identified?
KHAIRA: Yes.
HANNA: That happens in patients who have minimally metastatic disease. If you only have a malignant pleural effusion, or you have just small-volume metastasis, you may not find anything circulating. But for a patient who has gross metastatic disease, it is unusual to not find circulating tumor [ct] DNA.
KHAIRA: Yes, it’s interesting, because it was a large tumor, and I think one of the things in the future is using testing, such as FoundationOne, to screen [patients] for cancer. And it’s going to be interesting to see what percentage [of patients] don’t have anything [on the test], even if they have cancer.
HANNA: But that [type of] testing is [much] different [from] the testing that we do now. That testing would require a far deeper dive into genes and looking for many more genes than just the targeted genes that we look for in our current testing.
Plasma ctDNA testing for the purpose of detecting minimal residual disease, which is coming soon, is [much more] different than the commercial testing that you do looking for targetable mutations.
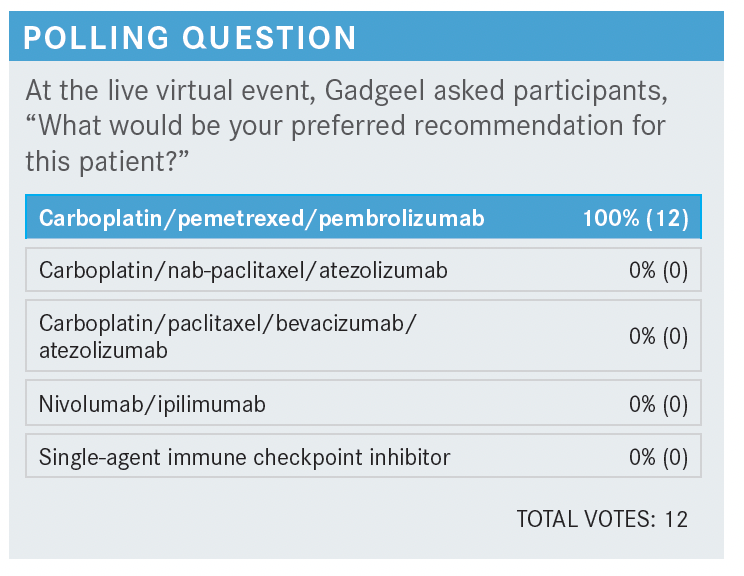
SINGH-ABRAHAM: I have used the nivolumab [Opdivo] plus ipilimumab [Yervoy] combination with and without chemotherapy, but I’ve been using it more so if I really want to avoid chemotherapy and I want a more immunotherapy-based regimen [because] the patients have brain metastasis or their PD-L1 [is] low. Since this patient had 10% PD-L1, I would favor carboplatin plus pemetrexed plus pembrolizumab.
GADGEEL: [When there is] brain metastasis, do you prefer to do nivolumab plus ipilimumab?
SINGH-ABRAHAM: I do, just for the hope that the immunotherapy crosses the blood-brain barrier and gives some efficacy results there.
GADGEEL: I’m not sure about the nivolumab plus ipilimumab data for patients with brain metastasis. I think that’s a good thought. [Dr Hanna,] are you aware of any data against using this combination in patients with brain metastasis?
HANNA: Well, as you know, these are monoclonal antibodies, huge proteins that [are not found in large concentrations] in the CNS [central nervous system]. However, they do have a physiological effect. We do know that single-agent PD-1 and PD-L1 inhibitors cause CNS responses. Perhaps the ipilimumab can cause responses in a different subset.
When we look at the CheckMate 227 [NCT02477826] data,3 we ask ourselves, “What is the role of ipilimumab, if any?” And it’s hard to find a role for ipilimumab in high PD-L1 expressors. Does it really add much? In the PD-L1 low expressors, particularly the 0, there is a suggestion that it may have benefit there. It’s not approved by the FDA for [patients with a PD-L1 score of] 0, although you could do chemotherapy plus nivolumab plus ipilimumab as well.
I don’t use a lot of ipilimumab. You counsel your patients that they should do generally well with immunotherapy, and if you get a single-agent checkpoint inhibitor, there’s [approximately] a 10% chance they’re going to need steroids to react to an immune-related AE [adverse event]. If you add ipilimumab, it’s 30%; and if you just do a single agent, it’s very unlikely your patient’s going to require hospitalization. If you add ipilimumab, 10% to 20% of those patients require some hospitalization at some point. I think we don’t have enough data to justify adding ipilimumab, except if the patient has a high-volume disease and is PD-L1 negative. [This is] where, frankly, carboplatin plus pemetrexed plus pembrolizumab does not perform very well, and maybe nivolumab plus ipilimumab could outperform it.
GADGEEL: I would add that the data were a little more impressive in squamous than in nonsquamous in that population, the [patients who are] PD-L1 negative, for nivolumab plus ipilimumab.
HANNA: You remember the original studies of nivolumab vs docetaxel [Taxotere] in the second line. There was an adenocarcinoma study and a squamous carcinoma study. In the squamous carcinoma study, if [a patient was] PD-L1 negative, nivolumab was no better than docetaxel. So there is a difference in histology with squamous carcinoma and if you’re PD-L1 negative.
KHAIRA: Going back to the brain metastasis, would atezolizumab be a better drug to use?
GADGEEL: I don’t know if we have any data on whether anti–PD-L1 drugs are better than anti–PD-1 drugs. I think [most comparisons are of] these drugs as immunotherapy combinations vs chemotherapy plus pembrolizumab, though I’m not certain. The way I think about it is that if a therapy is going to have a systemic effect, there is a reasonable chance that that therapy is going to have an effect in the brain, especially if the patient has asymptomatic brain metastasis. So if chemotherapy plus pemetrexed plus pembrolizumab is going to be efficacious in the extracranial disease, there’s a decent chance that it would also be effective in the brain.
MULHERIN: I probably do pembrolizumab alone more. I find that patients tend to be more tired after receiving 4 cycles of chemotherapy. I have continued the pemetrexed. I guess I’ve done that more if patients had a lower PD-L1, meaning they might not have as much of a benefit from pembrolizumab alone, but they can’t keep going on with pemetrexed forever. They’re going to have more fatigue, more anemia, and all kinds of other things. Several of my patients who are older are happy to get rid of the chemotherapy.
GADGEEL: I tend to continue pemetrexed, though I completely agree with you that patients get tired. I will say that I’ve noticed that ever since I’ve started using pembrolizumab every 6 weeks, particularly when I switched patients who are receiving every 3 weeks to every 6 weeks, I feel I’m seeing more immune-related AEs. Has anybody else noticed that?
HANNA: Yes. I think it causes more of that malaise.

Peers Discuss Role of Pola-R-CHP vs R-CHOP in Newly Diagnosed DLBCL
April 19th 2024During a Case-Based Roundtable® event, Haifaa Abdulhaq, MD discussed with participants whether the POLARIX trial influences their choice to use the pola-R-CHP as opposed to R-CHOP regimen for patients with newly diagnosed diffuse large B-cell lymphoma.
Read More
Powell Reviews Updated IO/TKI Data and AE Management in Endometrial Cancer
April 18th 2024During a Case-Based Roundtable® event, Matthew A. Powell, MD, discussed the case of a patient with advanced endometrial cancer treated with lenvatinib plus pembrolizumab who experienced grade 2 treatment-related hypertension.
Read More
Savona Discusses First-Line JAK Inhibition for Patients With Myelofibrosis at Risk of Anemia
April 17th 2024During a Case-Based Roundtable® event, Michael Savona, MD, and participants discussed the case of a patient with myelofibrosis and moderate anemia receiving JAK inhibitor therapy.
Read More
Biomarker Testing Paves the Way for Better Targeted Therapies in NSCLC
April 16th 2024At a live virtual event, Edward S. Kim, MD, MBA, discussed the evolving landscape of biomarker testing before making treatment decisions for patients with early-stage non–small cell lung cancer (NSCLC).
Read More