Batteries Beyond Lithium
UMD, Army Researchers Develop New Electrolyte to Bolster Rechargeable Tech, Bypass Raw Material Shortages
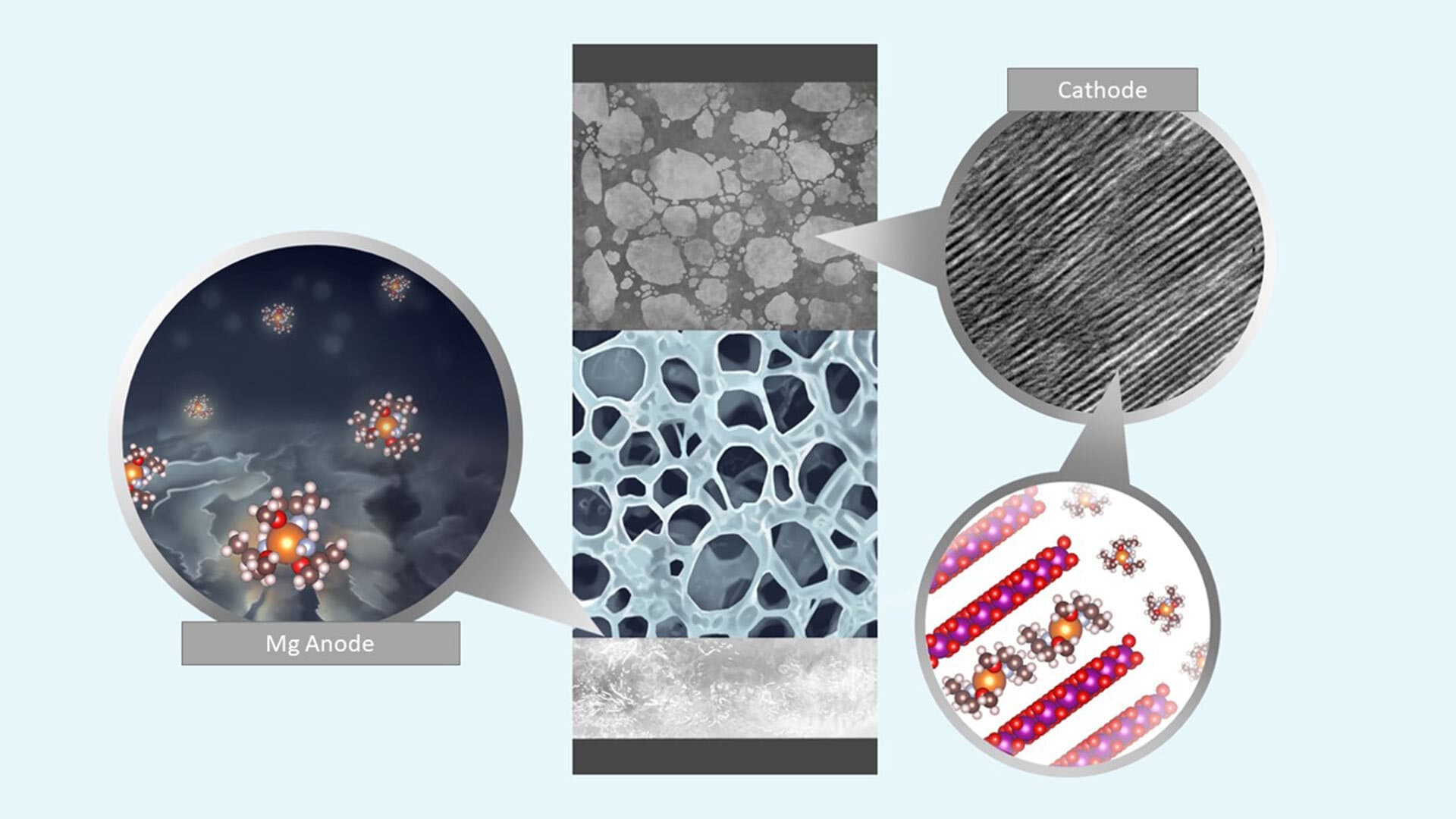
A UMD research team has developed a novel design strategy that incorporates a new class of solvents that could lead to magnesium metal batteries made of more-widely available base ingredients than lithium-ion batteries, while have equal or higher energy density.
Illustration by Nina Borodin, Singyuk Hou, Xiao Ji
Energy-packed lithium-ion batteries are helping reshape intelligent technologies that will one day make things easier, whether at home, at work or on a future battlefield, but there’s a problem: The sheer usefulness of the technology is driving worldwide demand for lithium-ion battery materials such as lithium, cobalt and nickel, posing the risk of a supply crunch.
A new breakthrough by University of Maryland researchers and the U.S. Army Research Laboratory may open up new possibilities for promising lithium-ion alternatives, such as rechargeable magnesium metal batteries. A detailed study about this novel electrolyte technology was published last week in the journal Science.
“Magnesium is substantially more abundant than lithium, which should meet the needs of the ever-growing battery market,” said Oleg Borodin, Army computational chemist.
Magnesium batteries could rival or even exceed lithium-ion in energy density, Borodin said. In addition, compared to lithium, magnesium is less prone to forming dendrites—metallic, tree-like structures that form during battery charging that experts have pinpointed as the main cause of safety concerns in lithium-ion batteries, including fires and explosions.
Despite these advantages, magnesium metal batteries still face many potential roadblocks. One is magnesium’s strong reaction with conventional electrolytes during battery operation; both electrodes in the battery must be compatible with this electrically conducting solution for the battery to attain a sufficient energy density. But as a material for anodes—the electrode through which electrical current flows out of a battery—magnesium tends to corrode the electrolyte and create a thick coating around the anode.
While similar coatings in lithium-ion batteries enable the diffusion of lithium-ions and protect the electrolyte from further decomposition, this coating instead blocks the magnesium plating and inhibits the necessary electrochemical reactions.
To address this problem, a UMD A. James Clark School of Engineering research team led by Chunsheng Wang, professor of chemical engineering and director of the Center for Research in Extreme Batteries, developed a novel design strategy that incorporated a new class of solvents.
To the research team’s surprise, the electrolyte design not only prevented the corrosion process, but it also dramatically increased the reaction dynamics of both the anode and the cathode (the electrode through which current flows in), boosting the battery’s overall performance.
“It’s the first time a magnesium battery theoretically reached a similar energy density as a lithium-ion battery,” Wang said.
The researchers also found they could apply the same design principle to other materials, not just magnesium.
According to Wang, the team plans to optimize the electrolyte and then scale up the concept into a large-scale power cell.