Multiple Combinations Demonstrate Efficacy and Provide Options for NSCLC
In a patient case reviewed by Gilberto De Lima Lopes, MD, a 59-year-old man presented with chest pain, cough, and dyspnea.
Gilberto De Lima Lopes, MD

Gilberto De Lima Lopes, MD led a Targeted Oncology Case-Based Roundtable event, in which a group of physicians discussed combination treatment options for a patient with non-small cell lung cancer.
Targeted OncologyTM: What do the National Comprehensive Cancer Network (NCCN) guidelines recommend for a patient such as this?
LOPES: The NCCN recommendations for patients with nonsquamous non-small cell lung cancer [NSCLC] for front-line testing include EGFR, ALK, and ROS1, which have become standard, and we have been doing for nearly a decade.1 Newer targets include BRAF, NTRK1/2/3, METex14 [MET exon 14] skipping mutation, and RET; hopefully, soon we will be asking for KRAS G12C, although that’s not in the guidelines. Testing should be conducted as part of a broad molecular testing so that you don’t waste material and get the results in a way that is clinically significant and useful. PD-L1 testing should be done as well. Again, the idea is that we should try to identify even those rare driver mutations for which we might have clinical trials or eventually drugs that may be approved. This is now considered a key component of standard of patient care.
The recommendation for liquid biopsy right now is if we have insufficient tissue to allow testing for all of the targets, we should either repeat the biopsy and/or do plasma testing.1 A lot of institutions around the country have moved on to do both at the same time, because if you do get a positive result and you find a target in the liquid biopsy, you gain 1 or 2 weeks in terms of being able to get your patient the drug. But that’s still not standard of care.
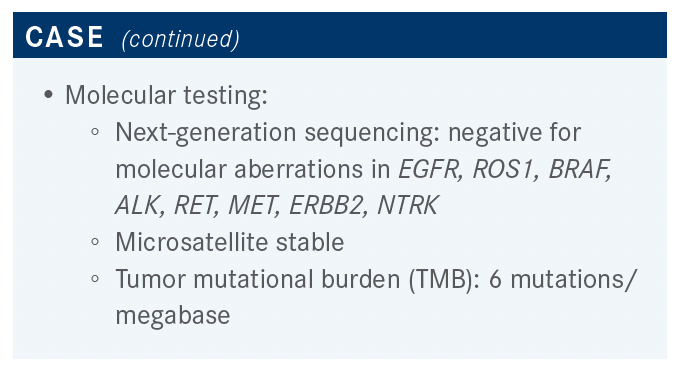
Please discuss the poll results and the options for this patient, knowing he is negative for any molecular aberrations.
Everybody voted for carboplatin/pemetrexed [Alimta]/ pembrolizumab [Keytruda], which tends to be the choice that is selected most often around the country—although, to be completely honest, pretty much every other option is also FDA approved and defendable. But this is exactly what I do most of the time, as well: carboplatin, pemetrexed, and pembrolizumab.
In the NCCN guidelines, for patients that have no driver mutations and PD-L1 expression between 1% and 49%, the preferred [category 1] option is a platinum agent plus pemetrexed plus pembrolizumab.1 But the NCCN guidelines also recommend several other options such as the IMpower150 regimen [NCT02366143], which was carboplatin/paclitaxel/bevacizumab [Avastin]/ atezolizumab [Tecentriq]. Carboplatin/albumin-bound paclitaxel/atezolizumab is another option, [as is] nivolumab [Opdivo]/ipilimumab [Yervoy] with 2 cycles of chemotherapy. NCCN also recommends nivolumab/ipilimumab without chemotherapy, or pembrolizumab without chemotherapy in certain cercumstances.1,2
How was the regimen the participants selected for this patient investigated in clinical trial?
In the KEYNOTE-189 study [NCT02578680], 616 patients were randomized 2:1 to receive platinum/pemetrexed/ pembrolizumab every 3 weeks for 4 cycles, followed by maintenance pemetrexed and pembrolizumab for a total of 2 years, vs standard chemotherapy in the control arm.3 The primary end points were OS [overall survival] and PFS [progression-free survival]. At 23.1 months follow-up, for OS there was an hazard ratio of 0.56, representing a 44% decrease in mortality when pembrolizumab [was added] to the platinum/pemetrexed backbone [95% CI, 0.45-0.70].4 At 12 months, we have 48% of patients alive vs 70%; at 24 months, 29% vs 45%, and the median goes up from 10.7 months to 22 months. This is for the ITT [intention-to-treat] population.
When the results are broken down by PD-L1 expression, those with a TPS [tumor proportion score] of less than 1%—even patients who had no expression—had a hazard ratio of 0.52 [95% CI, 0.36-0.74], and median survival improved from 10.2 to 17 months.4 At 24 months, 15% vs 38% of patients were alive, favoring the combination with added pembrolizumab. For those with a TPS between 1% and 49%, which is like the case we’re discussing, the median survival goes from 12 months to 21.8 months. At 24 months, instead of 33% of patients surviving, we have 44.3% survival for patients who got pembrolizumab in addition to platinum and pemetrexed. Finally, for patients with TPS of 50% or greater, median survival goes from 10 months to not reached, and at 24 months we have 40% vs 51.9% of patients being alive.
Which other studies have looked at pembrolizumab in NSCLC?
Let’s discuss the KEYNOTE-042 study [NCT02220894].4-6 Patients with a TPS of 1% and higher were randomized to receive pembrolizumab alone or chemotherapy alone. Chemotherapy could have been carboplatin plus pemetrexed or carboplatin plus paclitaxel. We included patients with both squamous and nonsquamous NSCLC, as long as they did not have any EGFR or ALK alterations. About 1200 patients were included in the study, and we saw that the greatest benefit of pembrolizumab alone was for patients with PD-L1 of 50% or greater [HR, 0.69; 95% CI, 0.56-0.85; P = .0003]. The median survival rates improved from 12.2 months to 20 months. In those patients with PD-L1 more than 1%, which was the ITT population of the trial, median OS improved from 12 months to 16.7 months. In those with PD-L1 greater than 20%, survival improved from 29.6 months to 40 months.
However, in the patient population with PD-L1 expression of 1% to 49%, we saw the survival curves crisscross, and we could not show that pembrolizumab was better.4-6 That was not initially planned; we did an exploratory analysis so that we could have a better idea of what [happened to] that population. The median OS for the PD-L1 of 1% to 49% was not worse with pembrolizumab, but it was not better, either.
Please discuss the IMpower150 trial results.
IMpower150 had 3 arms: [arm A was] atezolizumab plus carboplatin plus paclitaxel, [arm B was] atezolizumab plus carboplatin plus paclitaxel plus bevacizumab, [and arm C] was carboplatin plus paclitaxel plus bevacizumab for 4 to 6 cycles.7 The arm that we rarely speak about [was C], the atezolizumab plus carboplatin plus paclitaxel arm, because the benefit that we saw with the addition of atezolizumab was mostly in arm B in those who received bevacizumab as well. Median survival with the 4-drug combination went from 14.7 months to 19.2 months.2,7 The hazard ratio was 0.78, and this was statistically significant as well [95% CI, 0.64-0.96; P = .02].
What other combination therapies have shown efficacy in this setting?
Let’s review the results for patients with PD-L1 greater than 1% in the CheckMate 227 study [NCT02477826].8-10 The hazard ratio at the 30-month follow-up was 0.79, and that shows us the benefit of the nivolumab plus ipilimumab regimen vs chemotherapy alone [97.7% CI, 0.65- 0.96].10 Patients with less than 1% PD-L1 expression had the strongest signal for benefit, with a hazard ratio of 0.62 [95% CI, 0.49-0.79]. But because of the way the study was designed and presented to the FDA, this is not what we have in the approval for ipilimumab and nivolumab, and I would be hard-pressed to say that there’s anybody who uses this regimen for patients with no expression today.11 In RET, the expression between 1% and 49% was a study subgroup analysis, and the confidence interval crossed 1, so I’m not so sure about the benefit moving forward [HR 0.94; 95% CI, 0.75-1.18].9 I tend to prefer to using chemotherapy plus pembrolizumab but would still consider ipilimumab plus nivolumab for those patients who do not want chemotherapy. The treatment-related AEs [adverse events] with ipilimumab plus nivolumab using the 1 mg/kg dose of ipilimumab are tolerable, and this is something that we’re learning to use more and more.10,12,13 We really don’t see toxicity that is more than what we see with chemotherapy.
In CheckMate 9LA [NCT03215706], patients received ipilimumab plus nivolumab plus chemotherapy for 2 cycles vs chemotherapy alone, and the results again show that adding immunotherapy to chemotherapy improves survival.14,15
Median OS went from 10.9 months to 15.6 months, hazard ratio of 0.66 [0.55-0.80]. In those with PD-L1 expression of less than 1%, 1% to 49%, greater than 1%, and greater than 50%, there was a benefit in every subset. Regarding treatment-related AEs, there was more toxicity with the addition of ipilimumab plus nivolumab, but this is something that is very well managed, and we have certainly learned how to manage this over the past few years.
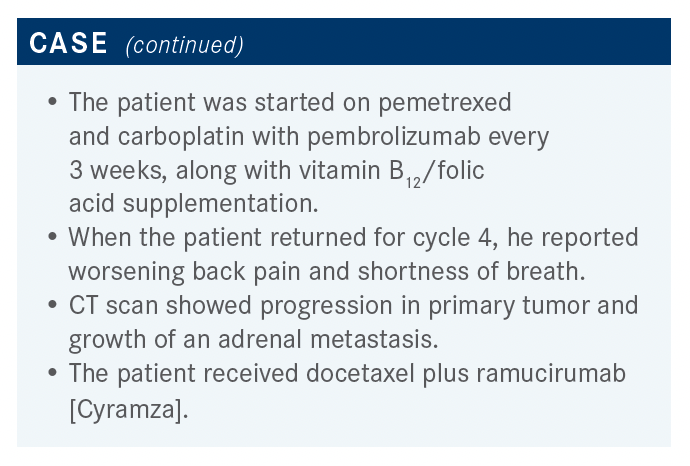
What are the data behind the patient’s newest treatment?
[In our case study] the patient received docetaxel plus ramucirumab. This was based on the REVEL study [NCT01168973], which was done before we had immunotherapy in the first line.16 Patients who had progressed after a platinum doublet were randomized to receive docetaxel at a dose that I, in particular, do not use routinely, which is 75 mg/m2 every 2 weeks, with or without ramucirumab 10 mg/kg. The results showed a small but real and significant—from a statistical point of view—[improvement in] median OS, from 9.1 months to 10.5 months [HR, 0.86; 95% CI, 0.75-0.98; P = .02]. The median PFS improved from 3 months to 4.5 months [HR, 0.76; 95% CI, 0.68-0.86; P < .0001]. Overall response rate was a little bit better, with 23% having a response in the doublet group vs 14% in the docetaxel group [OR, 0.76; 95% CI, 0.68- 0.86; P < .0001].
There was a sensitivity analysis and an exploratory analysis looking at patients with all histologies, or only adenocarcinoma, who remained on first-line therapy for less than 4 weeks, less than 8 weeks, and less than 12 weeks from the initiation of front-line therapy.17
But the trial did not include patients with [prior] immunotherapy. One of the results that we don’t discuss that often anymore, because all of us use ramucirumab, is that those patients that progressed in a quicker manner seemed to have a higher benefit. There was a hazard ratio of 0.40 and 0.44 for OS and PFS [with the doublet] vs 0.83 and 0.85 [with docetaxel].
The safety outcomes [were] nothing different from what we used to see [from] a VEGF/VEGFR inhibitor.17 Treatment-related AEs leading to dose adjustment were present in 33% who received the doublet vs 23% who received docetaxel. [Treatment-related AEs] leading to death [were the same between groups at] 5%, and serious AEs were very similar, at 43% and 42%, respectively.
REFERENCES:
1. NCCN. Clinical Practice Guidelines in Oncology. Non–small cell lung cancer, version 5.2021. Accessed September 9, 2021. https://bit.ly/3lycM8B
2. Socinski MA, Jotte RM, Cappuzzo F, et al; IMpower150 Study Group. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. Accessed September 12, 2021. doi:10.1056/NEJMoa1716948
3. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al; Keynote-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi:10.1056/NEJMoa1801005
4. Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. Accessed September 12, 2021. J Clin Oncol. 2020;38(14):1505-1517. doi:10.1200/JCO.19.03136
5. Lopes G, Wu YL, Kudaba I, et al. Pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol. 2018;36(suppl 18). doi:10.1200/JCO.2018.36.18_suppl.LBA4
6. Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab vs chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):P1819-P1830. doi:10.1016/S0140-6736(18)32409-7
7. Socinski MA, Jotte R, Cappuzzo F, et al. Overall survival (OS) analysis of IMpower150, a randomized Ph 3 study of atezolizumab (atezo) + chemotherapy (chemo) ± bevacizumab (bev) vs chemo + bev in 1L nonsquamous (NSQ) NSCLC. J Clin Oncol. 2018;36(suppl 15):9002. doi:10.1200/JCO.2018.36.15_suppl.9002
8. Ramalingam SS, Ciuleanu TE, Pluzanski A, et al. Nivolumab + ipilimumab vs platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: three-year update from CheckMate 227 part 1. J Clin Oncol. 2020;38(suppl 15):9500.
9. Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden.N Engl J Med. 2018;378(22):2093-2104. doi:10.1056/NEJMoa1801946
10. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
11. FDA approves nivolumab plus ipilimumab and chemotherapy for first-line treatment of metastatic NSCLC. FDA. Updated May 27, 2020. Accessed September 15, 2021.https://bit.ly/35tnarl
12. Yervoy. Prescribing information. Bristol Myers Squibb; 2021. Accessed September 15, 2021. https://bit.ly/3nFWpd2
13. Opdivo. Prescribing information. Bristol Myers Squibb; 2021. Accessed September 15, 2021. https://bit.ly/3hE8uf1
14. Reck M, Ciuleanu TE, Cobo Dols M, et al. Nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J Clin Oncol. 2020;38(suppl 15):9501. doi:10.1200/JCO.2020.38.15_suppl.9501
15. Paz-Ares L, Ciuleanu TE, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198-211. doi:10.1016/S1470-2045(20)30641-0
16. Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel vs placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665-673. Accessed September 15, 2021. doi:10.1016/S0140-6736(14)60845-X
17. Reck M, Paz-Ares L, Bidoli P, et al. Outcomes in patients with aggressive or refractory disease from REVEL: a randomized phase III study of docetaxel with ramucirumab or placebo for second-line treatment of stage IV non-small-cell lung cancer. Lung Cancer. 2017;112:181-187. doi:10.1016/j.lungcan.2017.07.038

Nogapendekin Alfa Plus Checkpoint Inhibition Improves Survival in NSCLC
April 25th 2024Following its recent FDA approval in non-muscle-invasive bladder cancer, nogapendekin alfa has also shown overall survival benefits in addition to checkpoint inhibitor therapy in patients with non-small cell lung cancer.
Read More
Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More