JOHAN WINNUBST: Tools to chart the neuronal landscape
The brain consists of a complex network of interconnected cells that have diverse properties. Classifying this diversity by grouping neurons with similar properties into neuronal ‘types’ has been a fundamental goal of neuroscience. However, collecting and integrating information across relevant cellular properties has remained challenging. The BRAIN Initiative Cell Census Network1 has taken up this challenge and created a cell atlas, focused mainly on a brain region called the primary motor cortex. The atlas incorporates molecular and functional data, as well as data related to physical form, for neurons in the brains of mice, non-human primates and humans (Fig. 1).
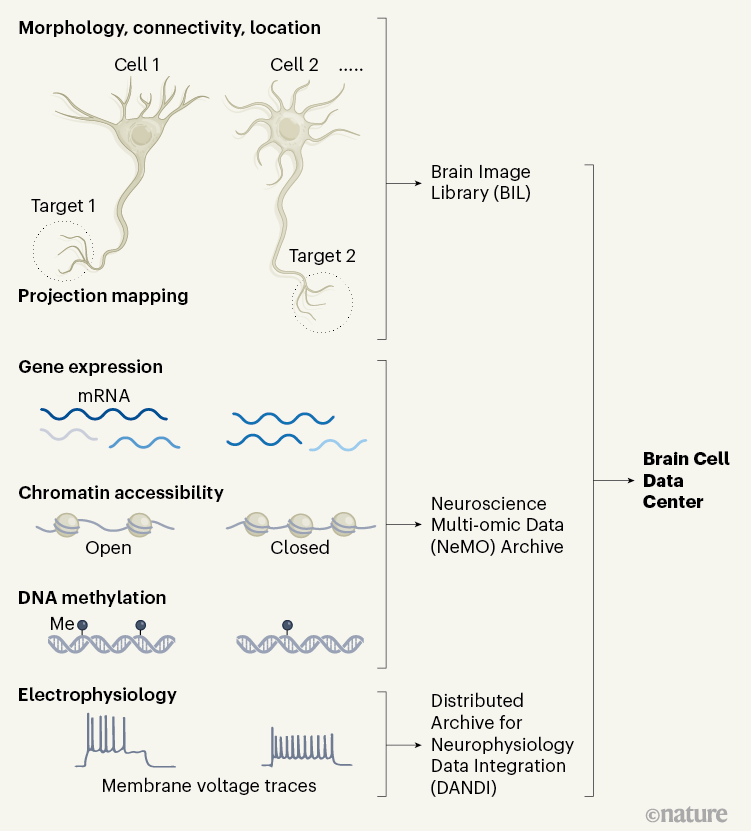
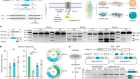
Figure 1 | Building a census of the cell types in the motor cortex of the brain. The BRAIN Initiative Cell Census Network (BICCN) investigated the diversity of cell types in the brain’s motor cortex in mice, non-human primates and humans1. The researchers categorized cells into different types on the basis of the cells’ morphology (shape), the pattern of their connections with other cells, their location and their connections with other brain regions (projection mapping). The consortium also sorted cells into types according to their gene expression and the patterns of epigenetic modification, such as the ‘opening’ of parts of the DNA–protein complex called chromatin to enable transcription, and the addition of methyl groups (Me) to DNA. The authors also characterized the cell types on the basis of their electrical properties (their electrophysiology). These data sets are available through various repositories and archives of the Brain Cell Data Center.
Rapid advances in single-cell RNA sequencing (scRNA-seq), a technique used to measure gene expression in individual cells, have already enabled major progress in characterizing neuronal diversity by generating gene-expression profiles for thousands of cells at a time. A key strategy of the BICCN was to use high-resolution scRNA-seq data to link information across various techniques. For example, M. Zhang et al.2 selected 258 genes whose expression was previously found to define different cell types. They used a technique called MERFISH to label cells expressing these marker genes in mouse brain tissue, allowing the spatial distribution of these cell types to be determined across the primary motor cortex.
Similarly, Berg et al.3 and Scala et al.4 used Patch-seq, a technique that combines recordings of the electrical properties of a cell with subsequent scRNA-seq and the morphological reconstruction of its 3D shape. Moreover, Liu et al.5 and Z. Zhang et al.6 profiled epigenetic modifications (genomic changes that affect gene expression without altering the DNA sequence) in cells whose projections were labelled by tracers injected into various brain regions. This enabled the authors to identify the long-range projection targets of different cell populations.
Read the paper: A multimodal cell census and atlas of the mammalian primary motor cortex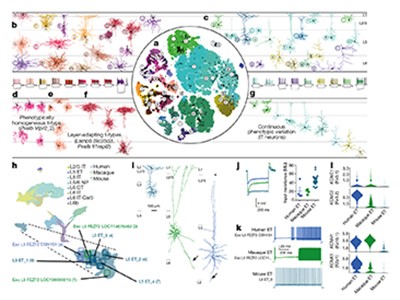
What are the origins of the vast neuronal diversity found in the mammalian brain during development and evolution? Several papers from the BICCN tackled this question by looking at the epigenetic modifications in cell types, as defined by patterns of gene expression, across species. For example, Bakken et al.7 and Yao et al.8 integrated gene-expression data with measurements of the abundance of methyl groups on the DNA of individual cells and the accessibility of DNA in the DNA–protein complex called chromatin. These measures indicate negative and positive regulation of gene transcription, respectively.
The studies by Bakken et al. and Yao et al. identified potential regulatory regions of the genome for different cell types. These regions are marked by genome stretches with highly accessible chromatin and low levels of DNA methylation, and include genes for regulatory transcription factors, and sites at which these factors bind to influence gene expression. This cell-type-specific enrichment of transcription factors and binding sites was remarkably conserved between species7 and could be used to generate genetic tools for studying the cell types further (see also Matho et al.9.
Although the technical progress made by the BICCN is impressive, further work to resolve the cellular diversity of the motor cortex is still needed. For example, Peng et al.10 present a library of individually reconstructed mouse neurons in which broader transcriptional subclasses are labelled. These reconstructions show how the axonal projections of neurons form complex connections across the brain. However, a full understanding of the relationship between a cell’s connectivity pattern and its genetic identity will require gene-expression profiles of individual cells to be linked to their axonal projections — perhaps using methods such as MERFISH.
Furthermore, the consortium’s findings emphasize the need for new conceptual and mathematical frameworks to understand the cellular diversity of the brain. For example, whereas cells in different classes usually have distinct characteristics, the finer-grained differences between cellular subtypes are often best described as a continuous gradient that correlates with a cell’s position in the brain2,4,5. It remains unclear how we should create a classification in which cells are simultaneously organized hierarchically (so that they can be separated into distinct, broad classes), continuously (in which subtypes can follow gradients on the basis of their genetic profile and other characteristics) and topographically (whereby diversity can correlate with cell location).
Although many of the BICCN’s techniques have been used before, this is the first time they have been applied in such a collaborative, high-throughput effort, providing an unprecedented view of the landscape of neuronal diversity. In addition, the considerable effort made by the consortium to make its data and software available to the research community (https://biccn.org) will ensure that this laudable achievement will continue to transform our understanding of the nature and origin of neuronal diversity.
SILVIA ARBER: Cell census will be a boon for future studies
The high diversity of cell types in the brain poses an enormous challenge to the neuroscience community. By building its cell-type census and atlas, the BICCN1 will accelerate efforts to unravel how neuronal cell types are defined, and how the cells are connected in networks and contribute to function.
The consortium focused on the motor cortex, a structure particularly well suited to such an in-depth investigation, for various reasons. First, the region extends neuronal projections directly to many other parts of the motor system and thus acts as a broadcasting device11,12 — but how it interacts with these other regions is poorly understood. Second, the motor cortex controls movement, and so understanding its cell types raises the possibility of linking them to function and behaviour. Third, from an evolutionary perspective, the region’s size, cell-type diversity and functions differ considerably across species11, an aspect that the BICCN specifically addressed through studies in mice, non-human primates and humans1,7.
A recipe book for cell types in the human brain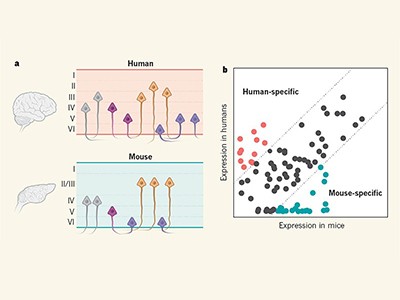
The consortium’s flagship paper highlights the main resources and findings generated by this enormous collaborative network1. Neuroscientists will have access to databases containing information about many aspects of the various cell types in the motor cortices of different species, including the cells’ gene expression, the molecular modifications that alter gene expression, the cells’ morphology and their electrophysiological properties (Fig. 1). The consortium has also generated many mouse strains in which key neuronal populations can be specifically targeted by particular manipulations — for example, to label them or control their activity9.
The papers accompanying the flagship paper prove the great benefits of mining data. Although the numbers of cell types identified using different methods vary1, future mining of combinations of the data sets provided could substantially strengthen conclusions on cell-type definitions, and aid efforts to understand their function and behaviour.
Cortical neurons have long been known to fall into two main categories: those communicating exclusively with other cortical neurons and those that also communicate with neurons outside the cortex. Some of the studies by the BICCN — including those by Matho et al.9, Peng et al.10 and Muñoz-Castañeda et al.13 — serve as starting points for future work to address how cell types in these categories are embedded and function in local cortical circuits, in loops with other structures in the brain, such as the basal ganglia and thalamus, and in long-range pathways to other regions of the cortex, brainstem and spinal cord. Understanding these cell types and their interactions will help to establish how they contribute to the learning, control and execution of movement, and how actions unfold at the systems level.
A decade of questions about the fluidity of cell identity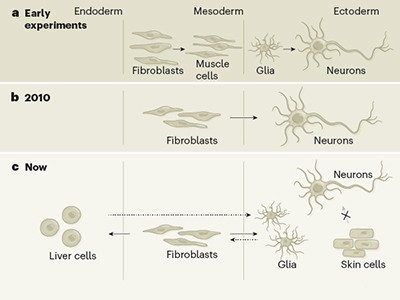
The BICCN papers present snapshots of the identities of adult neurons that — because they are, for the most part, not replaced throughout life — each have their own history. This includes the neurons’ developmental trajectory to maturation and individual experience up to their analysis, including modifications that occurred during learning and as a result of their plasticity (their dynamic ability to change their activity and connection properties). As previously shown in the spinal cord14, the genetic programs that operate during development are central to defining the basic properties of neuronal cell types, including functional attributes such as the neurotransmitter molecules they release, or their firing properties.
The genes that constitute these developmental programs can serve as markers of different neuronal types, and work by the BICCN (for example, by Matho et al.9) will advance researchers’ ability to target marker-defined cell types in mice. Later in life, however, experience influences gene expression, and can even lead to a switch in the type of neurotransmitter that a cell releases15. Future work should therefore determine the extent to which cell-type diversity reflects changes in gene expression due to experience, including learning and plasticity.
Furthermore, the BICCN papers also begin to compare cell types across species (see, for example, refs 3,7). This approach will provide insights into which neuronal cell types have evolved from others and which are evolutionarily new ‘add-ons’, and how they might contribute to evolutionarily new functions. Such work will be crucial to the design of cell-type-specific therapies for brain disorders because, although many principles of circuit function are conserved across species, any such interventions must be tailored to the human brain.
The BICCN papers represent a real treasure trove for future discovery. Of particular note are explorations that lead to a granular understanding of how the motor cortex helps to control and modulate many forms of movement. Neuroscience has indeed progressed a long way from the observations made around 150 years ago that electrical stimulation of a region in the cortex can elicit movement16.

 Read the paper: A multimodal cell census and atlas of the mammalian primary motor cortex
Read the paper: A multimodal cell census and atlas of the mammalian primary motor cortex
 A decade of questions about the fluidity of cell identity
A decade of questions about the fluidity of cell identity
 A recipe book for cell types in the human brain
A recipe book for cell types in the human brain