KRd-ASCT Improves Long-Term PFS in Multiple Myeloma
Promising survival results were shown in the FORTE trial of carfilzomib plus lenalidomide, and dexamethasone induction/consolidation with autologous stem cell transplant and maintenance with carfilzomib-lenalidomide in patients with multiple myeloma.
Significantly prolonger progression-free survival survival (PFS) rates were observed for carfilzomib (Kyprolis)- lenalidomide (Revlimid)-dexamethasone induction/consolidation with autologous stem cell transplant (KRd-ASCT) and maintenance with carfilzomib-lenalidomide (KR) when compared with KRd without ASCT (KRd12) and lenalidomide (R), respectively, in patients with multiple myeloma who had standard or high risk cytogenic abnormalities.
The data from the FORTE trial (NCT02203643) were presented at the 18th International Myeloma Workshop by investigator Roberto Mina, MD, assistant professor, Division of Hematology, Department of Molecular Biotechnologies and Health Sciences, University of Torino, Italy, and member of the medical monitoring team of the European Myeloma Network in Torino.
“Despite the limitations due to a small number of patients, the benefits of KRd plus transplant over KRd continuous without transplant and that of carfilzomib-lenalidomide versus lenalidomide alone as a maintenance were observed among all patient subgroups except those carrying amplification 1q,” Mina said during his presentation.
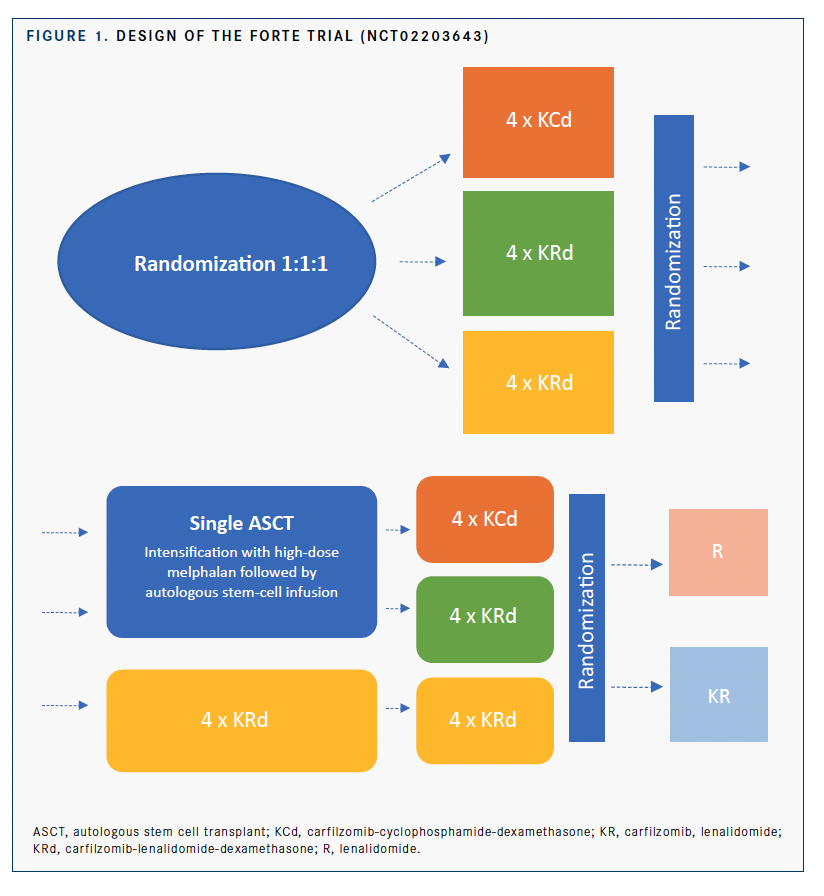
The trial included 396 patients who had received a new diagnoses of multiple myeloma, were transplant-eligible, and were younger than 65 years. Of the patients in this analysis, 243 were considered high risk, 105 were considered double hit, and 153 were considered standard risk.
“For this purpose, patients were stratified in 3 risk groups: standard risk if no chromosomal alteration was detected, high risk if at least 1 chromosomal alteration was detected, and double- hit myeloma in presence of at least 2 highrisk chromosomal abnormalities,” Mina said.
They were randomized to receive KRd-ASCT, carfilzomib-cyclophosphamide-dexamethasone (KCd) plus ASCT (KCd-ASCT), or KRd without ASCT (KRd12). After the second consolidation, they were again randomized to receive either KR or R maintenance therapy (FIGURE).
In the overall patient population, the KRd-ASCT arm had significantly prolonged progression-free survival compared with the KRd12 and KCd-ASCT arms. KR maintenance also had significantly prolonged PFS compared with R maintenance.
Among the high-risk patients, KRd-ASCT improved PFS compared with KRd12 (HR, 0.6; P = .04) and KCd-ASCT (HR, 0.57; P = .01), with 4-year PFS of 62%, 45%, and 45%, respectively.
In double-hit patients, KRd-ASCT improved PFS compared with KRd12 (HR, 0.53; P = .07) and KCd-ASCT (HR, 0.49; P = .03), with 4-year PFS of 55%, 31%, and 33%, respectively.
Similarly, with standard-risk patients, KRd-ASCT improved PFS when compared with KRd12 (HR, 0.47; P = .05) and KCd-ASCT (HR, 0.38; P = .01), with 4-year PFS rates of 80%, 67%, and 57%, respectively.
Looking at chromosomal abnormalities, there was a PFS benefit in patients with del17p, t(4;14), and 1q gain when receiving KRd-ASCT compared with KRd12. Patients with del1p saw greater benefit from KRd-ASCT andvKRd12 than KCd-ASCT. Patients with amp1qvhad the worst outcomes regardless of which treatment type they received.
KR improved PFS in all 3 groups compared with lenalidomide, with 3-year PFS survival rates of 90% versus 73% in standard-risk patients, 69% versus 56% in high-risk patients, and 67% versus 42% in double-hit patients.
There was more benefit with KR maintenance in patients with del17p, t(4;14), 1q gain, and del1p. Patients with amp1q again had the worst outcome and did not receive= any benefit from KR compared with R.
The results provide an effective option for high-risk patients, which is especially significant considering their clinical needs are currently unmet.
REFERENCE:
1. Mina R, Zamagni E, Fazio F, et al. Carfilzomib-based induction/consolidation with or without autologous transplant and lenalidomide (R) or carfilzomib-lenalidomide (KR) maintenance: efficacy in high-risk patients of the FORTE study. Presented at: 18th International Myeloma Workshop; September 8-11, 2021; Vienna, Austria.

Advances in Subsequent Therapies Shake Up Sequencing of ccRCC Treatment
April 25th 2024With the approval of belzutifan and other newer data for treating patients with recurrent renal cell carcinoma, the state of subsequent therapies is advancing beyond the reuse of frontline options with impacts on duration of response and quality of life.
Read More
Age, Disease Burden Are Factors in Early Use of Selinexor in Multiple Myeloma
April 22nd 2024During a Case-Based Roundtable® event, Jonathan L. Kaufman, MD, discussed treatment approaches and the tolerability of a selinexor-containing regimen in a patient with relapsed/refractory multiple myeloma in the first article of a 2-part series.
Read More