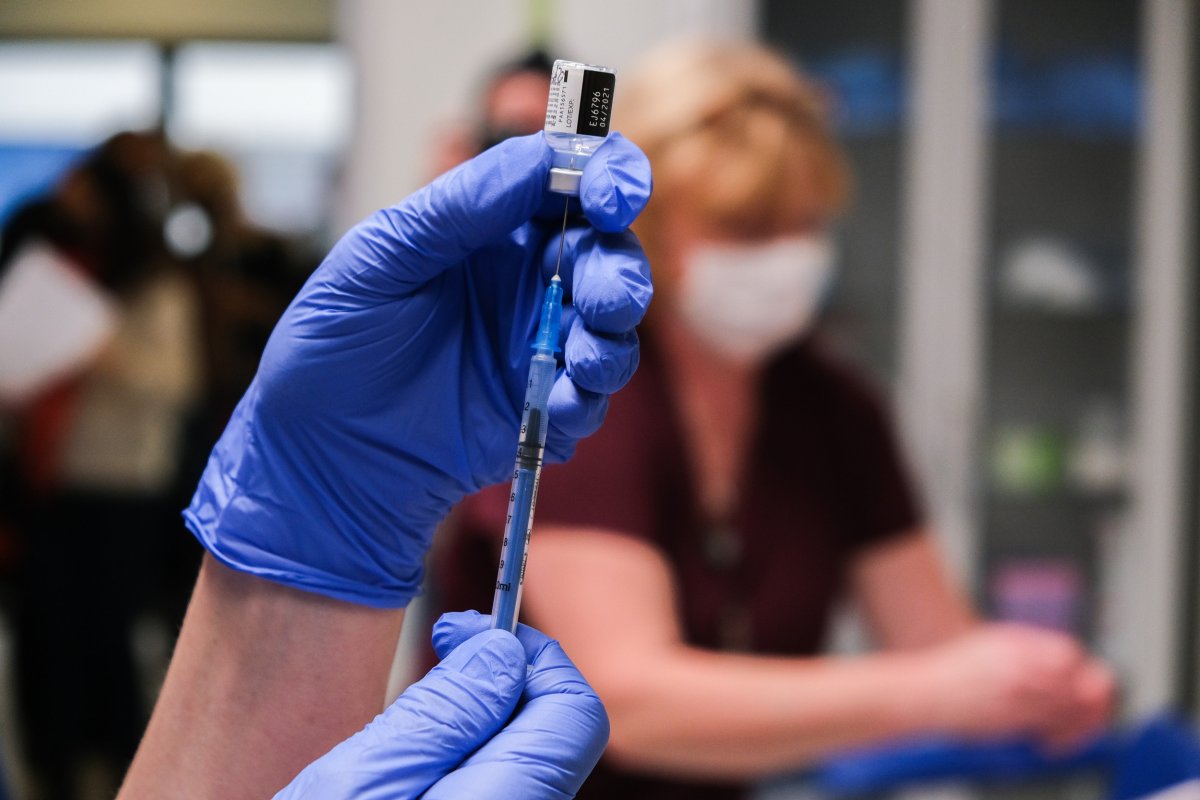
The U.S. Food and Drug Administration (FDA) on Wednesday announced it had authorized a booster dose of the Pfizer-BioNTech COVID vaccine for certain adults in the U.S.
It means an increased number of people will be eligible for a third shot of the Pfizer vaccine. Until now, additional shots were only for people with compromised immune systems.
However, the new booster shots will only be made available to people who have had the Pfizer vaccine, an FDA spokesperson told Newsweek. Otherwise, people would be mixing the vaccines.
The spokesperson said: "The authorization yesterday only applies to the Pfizer vaccine."
The confirmation comes after a federal health official said on Wednesday that there wasn't yet enough data to support giving a Pfizer booster shot to people who had previously had different kinds of vaccines, such as the Moderna or Johnson & Johnson jabs.
Doran Fink, a senior FDA vaccine official, said at an Advisory Committee on Immunization Practices meeting, according to The Wall Street Journal: "Data are not available to inform the interchangeability of a booster dose of one vaccine with the primary series of another vaccine."
Meanwhile Rochelle Walensky, director of the Centers for Disease Control and Prevention (CDC), which has endorsed the FDA's booster decision, said her agency was working to address similar recommendations for the other COVID vaccines currently available in the U.S.
Waiting for the Data
She said in an emailed press release: "We will address, with the same sense of urgency, recommendations for the Moderna and J&J vaccines as soon as those data are available."
This week's FDA approval means that a Pfizer booster shot will be allowed for people who are aged 65 or older; people who are aged 18 to 64 who are at high risk of severe COVID; and people aged 18 to 64 who are at risk of serious COVID complications due to being frequently exposed to it through their work or institution.
This means that groups such as health care workers, teachers, day care staff, grocery store workers and others are eligible for a Pfizer booster shot, acting FDA Commissioner Dr Janet Woodcock said in a statement.
Woodcock added: "As we learn more about the safety and effectiveness of COVID-19 vaccines, including the use of a booster dose, we will continue to evaluate the rapidly changing science and keep the public informed."
It comes after the Pfizer vaccine, with its new commercial name, Comirnaty, was approved by the FDA for preventing COVID in people aged 16 and over in the U.S. last month. Up until then, the vaccine had only been available under an emergency use authorization.
The FDA states that the Pfizer-BioNTech vaccine and Comirnaty have the same formulation and can be used interchangeably.
Uncommon Knowledge
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
Newsweek is committed to challenging conventional wisdom and finding connections in the search for common ground.
About the writer
To read how Newsweek uses AI as a newsroom tool, Click here.








