FDA panel overwhelmingly rejects approval of Pfizer vaccine booster shots for people 16 and older


A Food and Drug Administration advisory panel has voted against approving booster shots of the Pfizer-BioNTech COVID-19 vaccine for those 16 and older, just days before a Biden administration plan to administer additional doses to all Americans was set to begin.
An independent panel advising the FDA met on Friday to consider whether to endorse a third dose of Pfizer's COVID-19 vaccine for Americans 16 years and older. The committee voted 16 to 2 against doing so, The New York Times reports. The panel did, however, recommend booster doses for those 65 and older and at high risk for severe COVID-19, according to CNN.
Last month, the Biden administration announced a plan to begin offering COVID-19 booster shots to all Americans starting on Sept. 20, pending FDA and CDC approval. But it was later reported that the administration might have to scale this plan back, as during a meeting with the White House, top health officials "warned that more time may be needed before enough data is in to recommend boosters for all adults," CNN wrote.
Subscribe to The Week
Escape your echo chamber. Get the facts behind the news, plus analysis from multiple perspectives.

Sign up for The Week's Free Newsletters
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
From our morning news briefing to a weekly Good News Newsletter, get the best of The Week delivered directly to your inbox.
During the Friday meeting of the FDA advisory panel, committee member Dr. Michael G. Kurilla said that "it's unclear that everyone needs to be boosted, other than a subset of the population that clearly would be at high risk for serious disease," per the Times.
The Biden administration previously faced criticism for announcing its planned timeline to administer booster shots before they had been approved for all Americans.
"What I do think was backwards and not helpful was that the White House made an announcement with a certain date before really all the data had come in," former FDA Chief Scientist Jesse Goodman told CNN, "before [the] FDA had a chance to review it, and before there was this public discussion that we're now going to have."
Create an account with the same email registered to your subscription to unlock access.
Sign up for Today's Best Articles in your inbox
A free daily email with the biggest news stories of the day – and the best features from TheWeek.com
Brendan worked as a culture writer at The Week from 2018 to 2023, covering the entertainment industry, including film reviews, television recaps, awards season, the box office, major movie franchises and Hollywood gossip. He has written about film and television for outlets including Bloody Disgusting, Showbiz Cheat Sheet, Heavy and The Celebrity Cafe.
-
 The week's best photos
The week's best photosIn Pictures Playful goslings, an exploding snowman, and more
By Anahi Valenzuela, The Week US Published
-
 What is rock flour and how can it help to fight climate change?
What is rock flour and how can it help to fight climate change?The Explainer Glacier dust to the rescue
By Devika Rao, The Week US Published
-
 Crossword: April 19, 2024
Crossword: April 19, 2024The Week's daily crossword puzzle
By The Week Staff Published
-
 Texas dairy worker gets bird flu from infected cow
Texas dairy worker gets bird flu from infected cowSpeed Read The virus has been spreading among cattle in Texas, Kansas, Michigan and New Mexico
By Peter Weber, The Week US Published
-
 Dengue hits the Americas hard and early
Dengue hits the Americas hard and earlySpeed Read Puerto Rico has declared an epidemic as dengue cases surge
By Peter Weber, The Week US Published
-
 Covid four years on: have we got over the pandemic?
Covid four years on: have we got over the pandemic?Today's Big Question Brits suffering from both lockdown nostalgia and collective trauma that refuses to go away
By Chas Newkey-Burden, The Week UK Published
-
 US bans final type of asbestos
US bans final type of asbestosSpeed Read Exposure to asbestos causes about 40,000 deaths in the U.S. each year
By Peter Weber, The Week US Published
-
 The hollow classroom
The hollow classroomOpinion Remote school let kids down. It will take much more than extra tutoring for kids to recover.
By Mark Gimein Published
-
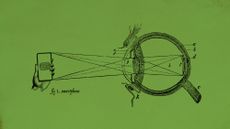 Excess screen time is making children only see what is in front of them
Excess screen time is making children only see what is in front of themUnder the radar The future is looking blurry. And very nearsighted.
By Devika Rao, The Week US Published
-
 Covid-19: what to know about UK's new Juno and Pirola variants
Covid-19: what to know about UK's new Juno and Pirola variantsin depth Rapidly spreading new JN.1 strain is 'yet another reminder that the pandemic is far from over'
By Arion McNicoll, The Week UK Published
-
 Long-term respiratory illness is here to stay
Long-term respiratory illness is here to stayThe Explainer Covid is not the only disease with a long version
By Devika Rao, The Week US Published