A Changing Tide for Patients With Myelofibrosis
The hallmarks of myelofibrosis—including clonal myeloproliferation, bone marrow fibrosis, anemia, splenomegaly, and constitutional symptoms—are associated with risk of morbidity and mortality; however, the recent advent of novel combinations and sequencing strategies have built on the foundation of care established with JAK2 inhibitors.
Pankit Vachhani, MD

Patients with myelofibrosis, a subclassification of Philadelphia chromo-some–negative myeloproliferative neoplasms (MPN), often present with symptom burden that excludes them from curative options, specifically transplant. The hallmarks of myelofibrosis—including clonal myeloproliferation, bone marrow fibrosis, anemia, splenomegaly, and constitutional symptoms—are associated with risk of morbidity and mortality; however, the recent advent of novel combinations and sequencing strategies have built on the foundation of care established with JAK2 inhibitors.1
Although investigators have observed progress in achieving clinical benefit for patients, efforts are needed to address lingering symptoms as well as progression on or resistance to standard-of-care therapy with ruxolitinib (Jakafi) for patients with intermediate- or high-risk disease and who are ineligible for transplant.
“In the field of myelofibrosis, there are several unmet needs, one being the improvement of overall survival in a setting of prior JAK2 inhibitor use and failure to it,” said Pankit Vachhani, MD, an assistant professor at the University of Alabama at Birmingham School of Medicine. “In the second-line space in myelofibrosis, we don’t have good options, [and] several clinical trials are ongoing to assess various new drugs [Table].”
Table. Ongoing Clinical Trials of JAK Inhibitors in Myelofibrosis
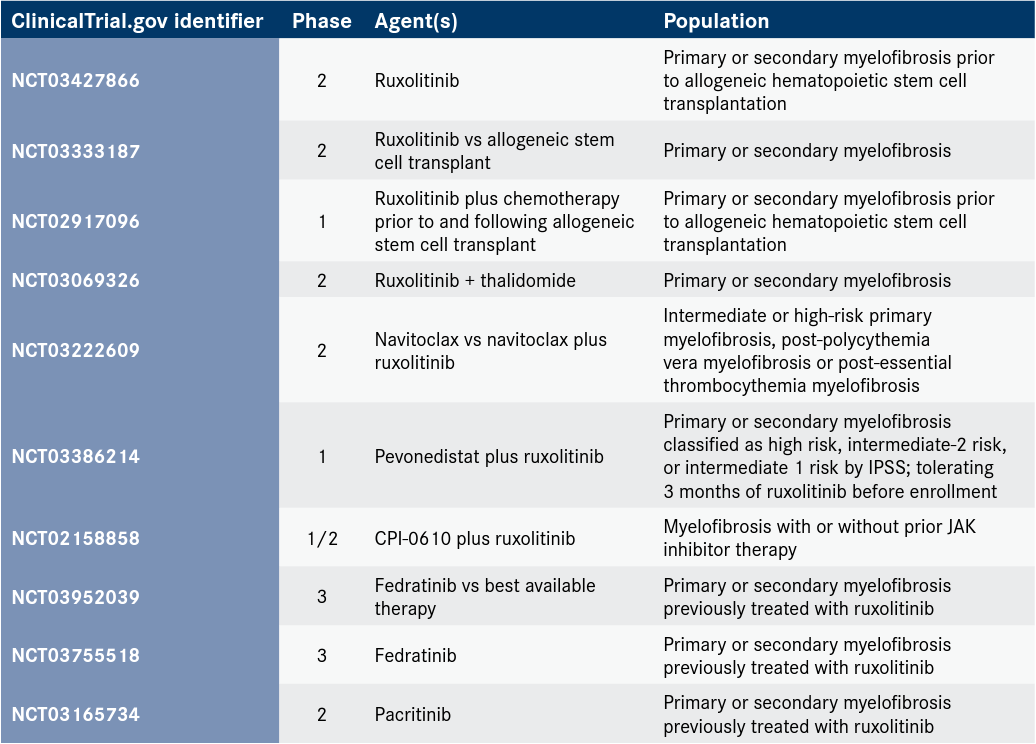
The management of anemia and thrombocytopenia represent 2 other areas of unmet need, according to Vachhani. “Thrombocytopenia, as one knows, is a risk factor for worse outcomes. But in the case of patients with myelofibrosis, and especially [those with] a platelet count of less than 50 x 109/L, for example, there aren’t any good treatment options,” he said. “[Additionally,] anemia can sometimes prevent physicians from using the most optimal dose of JAK inhibitor that they would like. It is a burden on the patients [because of the] transfusion requirement and symptoms such as fatigue, dyspnea, head-aches, and tiredness generally. Improving anemia therapeutically, [with or without] transfusion, would help improve the field and optimize the treatment options in the form of better dosing of JAK inhibitors or other treatment options.”
Building on Success With Ruxolitinib
Ruxolitinib was approved by the FDA for the treatment of patients with intermediate- or high-risk myelofibrosis based on data from the phase 3 COMFORT-I (NCT00952289) and COMFORT-II (NCT00934544) trials.2 Investigators of COMFORT-I compared ruxolitinib vs placebo in JAK inhibitor–naïve patients with myelofibrosis. Investigators of COMFORT-II compared ruxolitinib vs best alternative therapy in JAK inhibitor–naïve patients. Both trials met the primary end point of patients achieving greater than or equal to a 35% reduction from baseline in spleen volume vs placebo at 42% with ruxolitinib (n = 155) vs less than 1% with placebo (n = 154) in COMFORT-I (P < .0001) and 29% with ruxolitinib (n = 146) vs 0% with placebo (n = 73) in COMFORT-II (P < .0001).
In 2019, the FDA approved the JAK-2/FLT-3 inhibitor fedratinib (Inrebic) patients with intermediate- or high-risk myelofibrosis based on data from the JAKARTA trial (NCT01437787), in which 37% (n = 35/96) of patients treated with the recommended 400-mg dose achieved at least a 35% reduction in spleen volume vs 1 patient who experienced a response following treatment with placebo (n = 96; P < .0001).3
Investigators are exploring the long-term efficacy and safety of fedratinib in the phase 3, single-arm FREEDOM trial (NCT03755518) and the phase 3 FREEDOM2 trial (NCT03952039) vs best available care.
The clinical utility of ruxolitinib and fedratinib are challenged by compounding symptom burden and the development of JAK inhibitor–induced adverse effects, such as anemia. Further, patients who initially demonstrate a response to treatment will develop resistance to therapy after 2 to 3 years.1 For example, in the COMFORT-I and COMFORT-II trials approximately 50% of patients discontinued therapy by year 3 and 75% of patients discontinued by year 5. Indicators of suboptimal response to ruxolitinib may include failure to achieve minimum clinical improvement, or an increase in the severity of anemia, thrombocytopenia, or neutropenia within the first 4 months of therapy.
Ruben Mesa, MD

The "Add On" Effect
The JAK/STAT pathway has long been recognized as the central therapeutic target for myelofibrosis; however, investigators are looking to other viable options as a better understanding of escape mechanisms comes into focus. This includes “add-on” approaches, in which investigators are rechallenging patients whose disease exhibits resistance or failure to ruxolitinib, with the addition of new agents plus ruxolitinib to reignite response.
Pelabresib
BET and JAK inhibitors in combination have shown efficacy in preclinical humanized mouse models and may be a therapeutic strategy for myelofibrosis.4 Investigators hypothesize that the novel BET inhibitor pelabresib (formerly known as CPI-0610) in combination with ruxolitinib may circumvent treatment-related anemia, enhance splenic response, and address symptoms.
Results of the phase 2 MANIFEST trial (NCT02158858) showed that pelabresib was an effective treatment for patients with myelofibrosis in different clinical settings including as monotherapy for patients who are refractory, intolerant, ineligible, or are no longer receiving ruxolitinib (arm 1); as an additional therapy for patients being treated with ruxolitinib who are not adequately controlled (arm 2); and as a first-line treatment in combination with ruxolitinib for patients who have not received prior treatment with a JAK inhibitor (arm 3).5
In arm 1, seven of 23 (30%) evaluable patients who were not transfusion dependent at baseline achieved reduction in spleen volume at 24 weeks. Of the patients from arm 2 who received pelabresib as addition to ruxolitinib, 6 of 21 (29%) evaluable non-transfusion–dependent patients achieved reduction in spleen volume at 24 weeks. Findings from arm 3 included 63 evaluable patients and response was 67% (95% CI, 54%-78%) at 24 weeks.
The agent is under investigation in the phase 3 MANIFEST-2 trial (NCT04603495) in combination with ruxolitinib compared with ruxolitinib alone.
Navitoclax
Early efficacy has also been demonstrated with the addition of navitoclax to ruxolitinib in findings from the phase 2 REFINE trial (NCT03222609). Navitoclax, a small molecule targeting the BCL2 family of apoptotic receptors, including BCL-XL, may help overcome resistance to JAK2-targeted therapy based on results of preclinical models. Specifically, the inhibition of BCL-XL may result in cell death for JAK2-mutated cells and may prevent fibrosis in the bone marrow.6
Investigators of the REFINE trial enrolled patients who did not respond to ruxolitinib after at least 12 weeks of treatment were enrolled to receive the combination and were assessed for splenic volume reduction of at least 35%.7 At week 24, investigators observed splenic volume reduction in 27% of patients (n = 9/34) who had received at least 1 dose of the combination. Further, a reduction of 50% or greater in total symptom score through week 24 was reported for 6 of 20 patients (30%) and elicited bone marrow fibrosis improvements of at least 1 grade at any time in 29% of 34 patients.7
Investigators had a dual objective in the evaluation of findings from the study in that they sought to evaluate whether the presence of mutations in the high-molecular risk (HMR) category (ASXL1, EZH2, SRSF2 and IDH1/2) affected clinical outcomes. Mutational analysis was conducted at baseline and week 24, including next-generation sequencing with a 54-gene assay of variant allele frequency (VAF) in peripheral blood samples. Nineteen patients (58%) had mutations in HMR genes and, notably, 5 of the 9 patients (56%) who achieved splenic volume reduction at week 24 had HMR mutations.7
Investigators are exploring the efficacy of navitoclax and ruxolitinib as a potential first-line option for the treatment of myelofibrosis. The combination is being assessed vs ruxolitinib plus placebo in adults with primary or secondary MF who have not previously received a JAK2 inhibitor in the phase 3 TRANSFORM-1 trial (NCT04472598).8 The TRANSFORM-2 study (NCT04468984) is evaluating the combination vs best available therapy for patients with relapsed or refractory myelofibrosis.
Symptom Management Beyond the First Line
In later stages of development, investigators are setting their sights on 2 JAK2 inhibitors— momelotinib and pacritinib—that may provide more options for patients who experience treatment-related anemia and thrombocytopenia. “There are some important potential differentiators with the JAK inhibitors that are still in development,” said Ruben A. Mesa, MD, director of the Mays Cancer Center at UT Health San Antonio MD Anderson Cancer Center in Texas.
Momelotinib
Momelotinib, a JAK1/2 and ACVR1 inhibitor, may represent an option for patients with disease-related anemia. In long-term survival data, the agent conveyed a treatment benefit for patients with myelofibrosis regardless of prior JAK inhibitor therapy treated in the SIMPLIFY-1 (NCT01969838) and SIMPLIFY-2 (NCT02101268) trials. In addition to prolonging overall survival (OS), the agent had similar splenic response compared with ruxolitinib and reduced the transfusion burden and improved anemia.
“Data suggest that activity with momelotinib inhibits hepcidin, which may create a state of anemia, chronic disease, and inflammation,” Mesa said.
In SIMPLIFY-1, patients who had no prior JAK inhibitor were randomized to momelotinib monotherapy or ruxolitinib. At 4.5 years follow-up, the median OS was not reached for patients who started on momelotinib therapy and was 53.1 months for those who were assigned to ruxolitinib but crossed over to momelotinib. Patients enrolled in SIMPLIFY-2 had prior ruxolitinib treatment and were randomized to momelotinib monotherapy or best available care. The median OS was 34.3 months for those in the momelotinib arm vs 37.5 months in the crossover arm, which investigators reported as the “best OS in the previously ruxolitinib-treated setting.”9
Investigators are evaluating the agent in the MOMENTUM trial (NCT04173494) to determine if the agent, when administered after primary treatment with a JAK inhibitor, can continue to exhibit efficacy in reducing disease-related symptoms, the need for blood transfusions, and reverse splenomegaly.9
Pacritinib
Thrombocytopenia is a dose-limiting toxicity associated with JAK inhibitors, such as ruxolitinib and fedratinib. The available JAK inhibitors are not approved for individuals with a platelet count of less than 50,000.
To address this treatment gap, investigators are evaluating pacritinib, a JAK1/2 inhibitor, in this patient population. The agent, which has demonstrated efficacy in for patients with severe thrombocytopenia across trials, is currently under priority review for FDA approval. The application was based on findings from the phase 3 PERSIST-1 (NCT01773187) and PERSIST-2 (NCT02055781) trials, as well as data from the phase 2 PAC203 t rial (NCT03165734).10
Patients enrolled to the PERSIST-2 trial received pacritinib at a twice-daily dose of 200 mg (n = 74), and 29% experienced a reduction in spleen volume of at least 30% vs 3% of those who were given best available therapy (n = 72), which included ruxolitinib. Moreover, 23% of patients experienced a reduction in total symptom scores of at least 50% vs 13% of those given best available therapy.11
Data from the PERSIST-1 study showed that at 24 weeks, 19.1% of patients in the pacritinib arm (n = 220) experienced a 35% or greater reduction in spleen volume vs 4.7% of those in the best available therapy arm (n = 107; P = .0003). Of note, there was platelet count threshold was included for enrollment; however, 32% of patients had levels under 100,000 μL and 16% had levels under 50,000 μL.12
The phase 3 PACIFICA study (NCT03165734), which has opened as an amendment to the PAC203 trial, is recruiting patients with myelofibrosis and severe thrombocytopenia to evaluate pacritinib as first- or second-line treatment following JAK inhibition.
“Having application in this patient population sets pacritinib apart from other available treatments,” Mesa said.
Luspatercept
Another strategy to combat anemia is with luspatercept-aamt (Reblozyl). The agent was approved in 2020 for the treatment of adult patients with very low- to intermediate-risk MDS with ring sideroblasts or with myelodysplastic/myeloproliferative neoplasms with ring sideroblasts and thrombocytosis of anemia failing an erythropoiesis stimulating agent who require 2 or more red blood cell units over 8 weeks. The decision was based on data from the MEDALIST trial (NCT02631070).13
“The biggest change, compared with the [National Comprehensive Cancer Network] guidelines [for MPNs] from 2020, is the addition of the luspatercept,” Vachhani said.14 The agent is under investigation for patients with myelofibrosis on concomitant JAK2inhibitor therapy who require red blood cell transfusions vs placebo in the phase 3 INDEPENDENCE trial (NCT04717414).
“It has been gratifying to see [these updates to the guidelines],” Mesa said. “The [NCCN] guidelines provide some framework for a practice that had been quite heterogeneous, particularly in the United States, where some utilized the last review article they read or the last board review they heard or an outdated chapter of the [Ronald] Hoffman textbook [Hematology: Basic Principles and Practice] to guide how they were treating patients with MDS,” Mesa said, adding that the guidelines have “helped to bring [treatment strategies] into greater alignment.”
References
- Harrison CN, Schaap N, Mesa RA. Management of myelofibrosis after ruxolitinib failure. Ann Hematol. 2020;99(6):1177-1191. doi:10.1007/s00277-020-04002-9
- Jakafi. Prescribing information. Incyte; 2020. Accessed September 3, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202192Orig1s019Rpllbl.pdf
- FDA approves fedratinib for myelofibrosis. FDA. August 16, 2019. Accessed September 3, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fedratinib-myelofibrosis
- Jiang Q, Jamieson C. BET’ing on dual JAK/BET inhibition as a therapeutic strategy for myeloproliferative neoplasms. Cancer Cell. 2018;33(1):3-5. doi:10.1016/j.ccell.2017.12.007
- Verstovsek S, Mascarenhas J, Kremyanskaya M, et al. CPI-0610, bromodomain and extraterminal domain protein (BET) inhibitor, as “add-on” to ruxolitinib, in advanced myelofibrosis patients with suboptimal response: update of MANIFEST phase 2 study. Presented at: 25th European Hematology Association Annual Congress; June 11-21, 2020. Abstract EP1083. Accessed September 3, 2021. bit.ly/3zfZJ0J
- Harrison C, Garcia JS, Mesa R, et al. Navitoclax in combination with ruxolitinib in patients with primary or secondary myelofibrosis: a phase 2 study. Presented at: 25th European Hematology Association Annual Congress; June 11-21, 2020. Abstract EP1081. Accessed September 3, 2021. bit.ly/2O88BDZ
- Pemmaraju N, Garcia JS, Potluri J, et al. The addition of navitoclax to ruxolitinib demonstrates efficacy within different high-risk populations in patients with relapsed/refractory myelofibrosis. Blood. 2021;136(suppl 1):49-50. doi:10.1182/blood-2020-136938
- Potluri J, Hard J, Masud AA, Hutti JE. A phase 3, double-blind, placebo-controlled, randomized study evaluating navitoclax in combination with ruxolitinib in patients with myelofibrosis (TRANSFORM-1). Blood. 2020;136(suppl 1):4. doi:10.1182/blood-2020-139758
- Mesa RA, Kiladjian JJ, Catalano JV, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor–naïve patients with myelofibrosis. J Clin Oncol. 2017;35(34):3844-3850. doi:10.1200/JCO.2017.73.4418
- Harrison CN, Vannucchi AM, Platzbecker U, et al. Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial. Lancet Haematol. 2018;5(2):e73-e81. doi:10.1016/S2352-3026(17)30237-5
- CTI BioPharma announces completion of rolling submission of new drug application (NDA) for pacritinib in myelofibrosis patients with severe thrombocytopenia. News release. CTI BioPharma Corp; May 31, 2021. Accessed September 3, 2021. prn.to/3z2jtVf
- Results of the PERSIST-1 phase III study of pacritinib (PAC) versus best available therapy (BAT) in primary myelofibrosis (PMF), post-polycythemia vera myelofibrosis (PPV-MF), or post-essential thrombocythemia-myelofibrosis (PET-MF). J Clin Oncol. 2015;(suppl 33):LBA7006. https://bit.ly/2SgFNHL
- FDA approves luspatercept-aamt for anemia in adults with MDS. FDA. Updated April 6, 2021. Accessed September 3, 2021. bit.ly/391vXSy




